Physiology Ch 16 p177-189 [4-25
advertisement

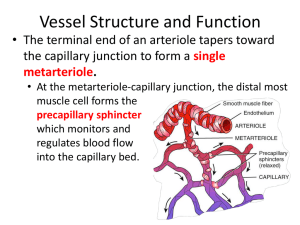
Physiology Ch 16 p177-189 Microcirculation and Lymphatic System -Transport of nutrients to tissues and removal of cell excreta occurs in the microcirculation -small arterioles control blood flow to each tissue, regulating their diameters based on the needs of each individual tissue -walls of capillaries are thin (single layer of permeable endothelial cells for H2O and nutrient exchange Structure of the Capillary System – each nutrient artery entering organ branches 6-8x before being called arterioles, which have diameters of 10-15um; arterioles branch 2-5x to form capillaries (5-9um) -arterioles are highly muscular, but terminal arterioles (metarterioles) do not have continuous muscular coat, rather smooth muscle fibers encircle the vessel at intermittent points -at the point where true capillary emerges from metarteriole, a smooth muscle fiber encircles the capillary, called the precapillary sphincter, which can open/close entrance to capillary -venules are larger than arterioles and have a weaker muscular coat, yet the pressure in venules is much less than that in arterioles, so venules can still contract despite the weak muscle -local conditions of the tissues control vessels for regulation of nutrients, metabolism, H ions, etc… Structure of Capillary Wall – wall is composed of unicellular layer of endothelial cells and is surrounded by a thin basement membrane on the outside of the capillary; total thickness of wall is 0.5um and the internal diameter is 4-9um Pores in the Capillary Membrane – 2 small passageways connect interior of capillary with exterior 1. intercellular cleft – thin-slit, curving channel that lies between adjacent endothelial cells a. each cleft is interrupted by short ridges of protein attachments that hold endothelial cells together, but between the ridges, fluid can percolate freely through cleft b. rate of water and ions is so rapid that they diffuse easily between interior and exterior capillaries through the intercellular clefts 2. plasmalemmal vesicles, also called caveolae form oligomers called caveolins that are associated with cholesterol and sphingolipids a. caveolae are thought to have endocytic functions and transcytosis of macromolecules across endothelial cells b. vesicles may form vesicular channels that move all the way through endothelial cell Special Types of Pores in Capillaries of Certain Organs 1. Brain: TIGHT junctions between capillary endothelial cells to only allow H2O, O2, and CO2 2. Liver: clefts between capillary endothelial cells are wide open so that almost all dissolved substances in plasma can pass from blood into the liver 3. GI: pores are midway between muscles and those of liver 4. Kidney: small oval windows called fenestrae penetrate through middle of endothelial cells so that tremendous amounts of very small molecular and ionic substances can filter through the glomeruli without having to pass through clefts between endothelial cells Flow of Blood in Capillaries – blood is not continuous in capillaries; instead it flows intermittently by turning on and off every few seconds/minutes; caused by vasomotion (intermittent contraction of metarterioles and precapillary sphincters) Regulation of Vasomotion – most impotant factor that affects the degree of opening and closing of metarterioles and precapillary sphincters is the concentration of O2 in the tissues -when rate of O2 usage by tissue causes tissue O2 to decrease, intermittent flow occurs more often, duration lasts longer to allow capillary blood to carry increased O2 to the tissue Average Function of the Capillary System – there are so many capillaries in tissues that the overall function becomes averaged – there is an average rate of blood flow in tissue capillary bed, an average capillary pressure, and an average rate of transfer of substances between blood of capillaries and surrounding interstitial fluid Exchange of H2O, Nutrients, and Other Substances Between Blood and Interstitial Fluid Diffusion Through Capillary Membrane – most important means of substance transfer between plasma and interstitial fluid is diffusion; as blood flows along capillary, H2O and dissolved particles diffuse back and forth through capillary wall, providing continual mixing between interstitial fluid and plasma -diffusion results from thermal motion of H2O molecules and dissolved substances in fluid Lipid Soluble Substances Can Diffuse Directly Through Cell Membranes of Endothelium – substances like O2 and CO2 are lipid soluble and can permeate all areas of capillary membrane H2O Soluble, Non-lipid-soluble Substances Diffuse Through Intercellular Pores in Endothelial Membrane – H2O, Na, Cl, glucose cannot pass through lipid membranes, and so they require the use of intercellular clefts for passage, which account for 1/1000 of the surface area of capillaries, but still tremendous diffusion takes place -rate of H2O diffusion is 80x larger than rate at which plasma flows linearly along capillary Effect of Molecular Size on Passage Through Pores – width of capillary cleftpore is 6-7nm (20x diameter of the H2O molecule) whereas plasma proteins are larger than the width of the pores, with Na, Cl, glucose, and urea having intermediate diameters -permeability of capillary pores for different substances varies according to diameters -glucose is 0.6x as permeable as water, whereas albumin is 0.001x as permeable as water -permeabilities vary depending on the organ being looked at Effect of Concentration Difference on Net Rate of Diffusion Through Capillary Membrane – net rate of diffusion through membrane is proportional to concentration difference of substance between both sides of the membrane; greater difference of concentrations, the greater the movement of substance -O2 concentration greater in capillaries, so O2 moves from blood to tissues, conversely for CO2 -rates of diffusion of most nutritionally important substances are so great that only slight concentration differences suffice to cause more than adequate transport between plasma and interstitial fluid Interstitium and Interstitial Fluid – 1/6th of body volume consists of spaces between cells, called the interstitium, the fluid between these spaces is called the interstitial fluid -the interstitium contains two major types of solid structures: collagen fibers and proteoglycans -collagen fibers extend long distances in interstitium and provide most tensional strength of tissues; proteoglycans are thin coiled or twisted molecules composed 98% hyaluronic acid and 2% protein; they form a mat of fine reticular filaments described as a brush pile “Gel” in the Interstitium – fluid in the interstitium is derived by filtration and diffusion from capillaries with the same constituents as plasma but lower protein concentration -interstitial fluid is trapped within proteoglycan filaments to cause a gel, called a tissue gel -due to the large number of proteoglycans, it is DIFFICULT for fluid to flow through gel, instead, fluid mainly diffuses through the gel by moving one molecule at a time driven by thermal motion -diffusion through a gel occurs about 95-99% as rapidly as it does through free fluid to allow rapid transport of nutrients and electrolytes Free Fluid in the Interstitium – almost all fluid is trapped in gel, and only small rivulets of free fluid and small free fluid vesicles are present, which can flow freely -amount of free fluid in normal tissues is slight (<1%) -when tissues develop edema, these pockets and rivulets expand tremendously until 50% of edematous fluid becomes freely flowing fluid independent of proteoglycan filaments Fluid Filtration Across Capillaries is Determined by Hydrostatic and Colloid Osmotic Pressures, as Well as Capillary Filtration Coefficient – hydrostatic pressure in capillaries forces fluid and substances through capillary pores into the interstitial spaces -osmotic pressure caused by plasma proteins (colloid osmotic pressure) causes fluid movement by osmosis from interstitial spaces into the blood -this pressure prevents significant loss of fluid volume from blood into the interstitial spaces -the lymphatic system returns to circulation the small amounts of excess protein and fluid that leak from blood into the interstitial spaces Hydrostatic and Colloid Forces Determine Fluid Movement Through Capillary Membrane – there are 4 forces that determine whether fluid moves out of blood into interstitium or vice versa (starling forces) 1. Capillary Pressure (Pc) – tends to force fluid OUTWARD through capillary membrane 2. Interstitial Fluid Pressure (Pif) – forces fluid INWARD through membrane when Pif is (+) and outward when Pif is (-) 3. Capillary plasma colloid osmotic pressure (Πp) – causes osmosis of fluid IN through membrane 4. Interstitial fluid colloid osmotic pressure (Πip) – causes osmosis of fluid OUT of membrane -The sum of these forces is called the net filtration pressure (NFP); if positive, there will be a net FILTRATION across the capillaries, if the sum of Starling forces is negative, there will be a net ABSORPTION of fluid from interstitial spaces into the capillaries NFP = Pc – Pif – Πp + Πif -NFP is slightly positive under normal conditions, resulting in net filtration of fluid across capillaries and into interstitial space -rate of filtration is also dictated by number and size of pores in each capillary, as well as the numer of capillaries; these are expressed together as the capillary filtration coefficient (Kf), which is a measure of capacity of capillary membranes to filter H2O for a given NFP (expressed in mL/min per mmHg filtration pressure) Filtration = Kf x NFP Capillary Hydrostatic Pressure – there are 2 methods to estimate capillary hydrostatic pressure: 1. Direct Micropipette Cannulation of Capillaries – given an average of 25mmHg in muscle and gut a. Microscopic glass pipette is thrust into capillary and a pressure is measured by a micromanometer to give 30-40mmHg in arterial ends of capillaries, 10-15mmHg in venous ends, and 25mmHg in the middle b. In some capillaries such as glomerular capillaries of kidney, pressures average 60mmHg; whereas the peritubular capillaries average only around 13mmHg 2. Indirect Functional Measurement – given a pressure averaging 17mmHg in tissues, called isograviemtric method, where a section of gut is held up by one arm of a gravimetric balance; blood is perfused through the vessels of gut wall a. When arterial pressure is decreased, resulting decrease in capillary pressure allows osmotic pressure of plasma proteins to cause absorption of fluid out of gut wall to make weight of the gut decrease, displacing the arm on the gravimetric balance b. To prevent weight decrease, venous pressure is increased to overcome effect of decreasing arterial pressure c. Capillary pressure is kept constant while decreasing arterial pressure and increasing venous pressure d. Gravimetric method gives lower value than direct method because capillary fluid filtration is not fully balanced with fluid reabsorption in most tissues Interstitial Fluid Hydrostatic Pressure – in subcutaneous tissue, interstitial fluid pressure is measured as a few mmHg less than atmospheric pressure, called negative interstitial fluid pressure -in other tissues surrounded by capsules, like the kidneys, the interstitial fluid pressure is positive -methods are (1) direct cannulation of tissues with micropipette, (2) measurement of pressure from implanted perforated capsules, and (3) measurement of pressure from a cotton wick inserted into tissue Measurement of Interstitial Fluid Pressure Using Micropipette – first pressures measured using micropipette ranged from -1 ot 2mmHg, but recently technology improved to give a -2mmHg value Measurement of Interstitial Fluid Pressure in Implanted Perforated Hollow Capsules – his method in normal loose subcutaneous tissue averages -6mmHg, but some also give -2mmHg Interstitial Fluid Pressures in Tightly Encased Tissues- some tissues such as brain, kidney, muscles, and sclera have tight encasements, causing interstitial fluid pressures to be positive, but are still less than pressures exerted on outsides of the tissues by their encasements -CSF pressure surrounding brain averages +10mmHg, whereas brain interstitial fluid is +5 mmHg -Kidney capsular pressure is +13mmHg and renal interstitial fluid pressures average +6mmHg An Average Value of Negative Interstitial Fluid Pressure in Loose Subcutaneous Tissue – true fluid pressure in loose subcutaneous tissue averages -3mmHg Pumping by Lymphatic System is Basic Cause of Negative Interstitial Fluid Pressure – lymphatic system is a scavenger system that removed excess fluid, proteins, and debris from tissue spaces -when fluid enters terminal lymphatic capillaries, the lymph vessel walls automatically contract for a few seconds and pump fluid into blood circulation; this creates slight negative pressure measured in interstitial spaces Plasma Colloid Osmotic Pressure – Proteins in Plasma Cause Colloid Osmotic Pressure – only molecules that fail to pass through pores of a semipermeable membrane exert osmotic pressure; proteins are the only dissolved constituents that do not readily pass through pores; those proteins are responsible for osmotic pressures across membrane. Normal values for Plasma Colloid Osmotic Pressure – colloid osmotic pressure of human plasma averages about 28mmHg; 19 caused by dissolved proteins and 9mm caused by Donnan effect (extra osmotic pressure caused by Na, K, and other cations held in plasma by the proteins) Effect of Different Plasma Proteins on Colloid Osmotic Pressure – plasma contains proteins with different weights: albumin (69000), globulins (140000), fibrinogen (400000); thus 1 gram of globulin has 50% of molecules as 1 g of albumin, and 1g of fibrinogen has 1/6th as many molecules as 1g of albumin -osmotic pressure is determined by the NUMBER OF MOLECULES rather than the mass, therefore, when corrected for number of molecules rather than mass, giving albumin as the major contributor -80% of total colloid pressure comes from albumin, 20% comes from globulins, and barely any comes from fibrinogen Interstitial Fluid Colloid Osmotic Pressure – small amounts of plasma proteins can leak through pores into the interstitial spaces through pores and by transcytosis in small vesicles; total quantity of protein in the entire 12L of interstitial fluid of the body is slightly greater than the total quantity of protein in the plasma itself, but because this volume is 4x the volume of plasma, the average protein CONCENTRATION of the interstitial fluid is only 40% that of plasma (3g/dL); average interstitial fluid colloid pressure is 8mmHg Exchange of Fluid Volume Through Capillary Membrane – average capillary pressure at arterial ends is 15-25mmHg greater than at venous ends; because of this, fluid “filters” out of capillaries at arterial ends, but is reabsorbed at the venous ends back into the capillaries -small amount of fluid “flows” through the tissues from the arterial ends of capillaries to venous ends Analysis if Forces Causing Filtration at Arterial End of Capillary – summation of forces at the arterial end of the capillary shows a net filtration pressure of 13mmHg, tending to move fluid OUT through the capillary pores -the 13mmHg filtration pressure causes 1/200th of plasma in flowing blood to filter out of arterial ends of capillaries into interstitial spaces each time the blood passes through the capillaries Analysis of Reabsorption at Venous End of Capillary – low BP at venous end of capillary changes the balance of forces in favor of absorption as follows: -thus, forces that cause fluid to move INTO the capillary, 28mmHg, is greater than the opposing reabsorption 21mmHg; the difference, 7mmHg is the net reabsorption pressure at the venous ends of capillaries -the reabsorption pressure is considerably less than filtration pressure at capillary arterial ends, but venous capillaries are more numerous and more permeable than arterial capillaries, so less reabsorption is required to cause inward movement of fluid -reabsorption pressure causes 9/10ths of the fluid that has filtered out of the arterial ends of capillaries to be reabsorbed at the venous ends; remaining 1/10th flows into lymph vessels and returns to blood Starling Equilibrium for Capillary Exchange – state of “near-equilibrium” exists in capillaries; where amount of fluid filtering out from arterial ends equals almost exactly fluid returned by reabsorption, with lymphatics taking up the slack; given by this chart showing starling equilibrium -pressures in arterial and venous capillaries are averaged to calculate mean functional capillary pressure, which happens to be 17.3 mmHg -the slight 0.3mmHg causes slightly more filtration into interstitial spaces than reabsorption, called net filtration, that must be returned by lymphatics -normal rate of net filtration in the ENTIRE BODY (not including the kidneys, is 2ml/min Filtration Coefficient – an average net imbalance of forces at capillary membranes of 0.3mmHg causes net fluid filtration in entire body of 2ml/min; expressing this for each mm of Hg imbalance, we find a net filtration rate of 6.67ml/min/mmHg for entire body, called the filtration coefficient -this coefficient can also be expressed for separate parts of the body in terms of rate of filtration per min per mmHg per 100g of tissue, therefore the coefficient of average tissue is 0.1/ml/min/mmHg/100g -differences in permeabilities of capillary systems of different tissues causes coefficient to vary -very small in brain/muscle, large in subcutaneous tissue, large in GI, EXTREME in liver + kidney where pores are either numerous or wide open Effect of Abnormal Imbalance of Forces at Capillary Membrane – if mean capillary pressure rises above 17mmHg, net force tending to cause filtration of fluid into tissue spaces rises -a 20mmHg rise in mean capillary pressure causes an increase in net filtration pressure from 0.3 to 20.3mmHg, resulting in 68x as much net filtration of fluid into interstitial spaces -lymphatics would have to take up 68x the normal flow, an amount that is 2-5 times too much for lymphatics to carry -as a result, fluid will accumulate in interstitial spaces and edema will result -if capillary pressure falls too low, net reabsorption will occur and blood volume will increase at the expense of interstitial fluid volume Lymphatic System – an accessory route through which fluid can flow from interstitial spaces into blood -lymphatics carry proteins and large particulate matter away from tissue spaces, neither of which can be removed by absorption directly into capillaries Lymph Channels of the Body – almost all tissues have special lymph channels that drain excess fluid directly from interstitial spaces; exceptions include superficial skin, CNS, endomysium of muscle, and bones. Even those tissues have prelymphatics through which interstitial fluid can flow -all lymph vessels from lower part of body can empty into the thoracic duct, which empties into the venous system at juncture of L internal jugular vein and L subclavian vein -lymph from the L side of head, the L arm, and part of chest also enters thoracic duct before it empties into the veins -lymph from R side of neck and head, R arm, and R thorax enters the R lymph duct which empties between the R subclavian vein and internal jugular vein Terminal Lymphatic Capillaries and Their Permeability – most of the fluid filtering from arterial ends of blood capillaries is reabsorbed into venous ends of blood capillaries, but 1/10th of fluid enters lymphatic capillaries and returns to blood through lymphatic system; only 2-3L/day -fluid that returns by lymphatics is important because it is high in molecular weight from proteins, and cannot be absorbed from tissues in any other way -a special structure of lymphatic capillaries helps absorb proteins unimpeded from interstitium -endothelial cells of lymphatic capillary attached by anchoring filaments to surround connective tissue -at the junctions of adjacent endothelial cells, the edge of one endothelial cell overlaps the edge of adjacent cell in such a way that the overlapping free edge is free to flap inward, forming a minute valve that opens to the interior of lymphatic capillary -interstitial fluid with particles can push the valve open and flow directly into lymphatic capillary, but it has difficulty leaving because any backflow causes the closure of the flap valve Formation of Lymph – lymph is derived from interstitial fluid that flows into lymphatics; as lymph first enters terminal lymphatics, it has almost the same composition as the interstitial fluid -protein concentration averages 2g/dL, and -in the liver, lymph formed has a higher concentration of 3-4g/dL; because 2/3rds of lymph is normally derived from liver/intestines, concentration of thoracic duct lymph is 3-5g/dL -lymphatic system is a major route for absorption of nutrients from GI tract, especially for absorption of all fats in food; after a fatty meal, thoracic duct lymph contains as much as 1-2% fat -large particles like bacteria can get into lymphatic capillaries from interstitial fluid and are cleared in the lymph nodes Rate of Lymph Flow – 100mL/hour of lymph flows through thoracic duct of human, and another 20mL flows into circulation each other through other channels, making flow 120mL/hr or 2-3L/day Effect of Interstitial Fluid Pressure on Lymph Flow – any factor that increases interstitial fluid pressure also increases lymph flow if the lymph vessels are functioning normally; they include: -elevated capillary hydrostatic pressure, decreased plasma colloid osmotic pressure, increased interstitial fluid colloid osmotic pressure, and increased permeability of capillaries -all of these cause a balance of fluid exchange at blood capillary membrane to favor fluid movement into the interstitium to increase interstitial fluid volume, interstitial fluid pressure, and lymph flow at the same time -when interstitial fluid pressure becomes 1-2mmHg greater than atmospheric pressure, lymph flow fails to rise any further at higher pressures -this results from fact that increasing tissue pressure not only increases entry of fluid into lymphatic capillaries but also compresses outside surfaces of larger lymphatics, impeding lymph flow -at higher pressures, these 2 factors balance exactly, so lymph flow reaches maximum lymph flow rate Lymphatic Pump Increases Lymph Flow – Valves exist in all lymph channels; when a collecting lymphatic or larger lymph vessel is stretched with fluid, smooth muscle in the wall of vessel automatically contracts -each segment of lymph vessel between successive valves functions as a separate automatic pump; even slight filling of a segment causes it to contract and fluid is pumped through the next valve into the next lymphatic segment; this fills the subsequent segment, which also contracts, and the process continues all along until the fluid is emptied into circulation Pumping Caused by External Intermittent Compression of Lymphatics – in addition to pumping caused by intrinsic contraction of lymph vessel walls, contraction of skeletal muscles, movement of body parts, pulsations of arteries adjacent to lymphatics, and compression of tissues by outside objects causes movement of lymph -lymphatic pump is very active during exercise, often increasing lymph flow 10-30fold -during rest, lymph flow is sluggish, almost 0 Lymphatic Capillary Pump – terminal lymphatic capillary is also capable of pumping lymph, in addition to the pumping by larger lymph vessels -walls of lymphatic capillaries are tightly adherent to surrounding tissues by means of anchoring filaments -each time excess fluid enters the tissue and causes swelling, the anchoring filaments pull on the wall of the lymphatic capillary and fluid flows into the terminal lymphatic capillary through the junctions between the endothelial cells -when tissue is compressed, pressure inside capillary increases and causes overlapping edges of endothelial cells to close like valves -therefore, pressure pushes the lymph forward into the collecting lymphatic instead of backward through cell junctions -lymphatic capillary endothelial cells also contain a few contractile actomyosin filaments, which can cause rhythmical contraction of lymphatic capillaries in the same way that many small blood and larger lymphatic vessels also contract rhythmically -primary factors controlling lymph flow are the interstitial fluid pressure and activity of lymphatic pump Role of Lymphatic System in Controlling Interstitial Fluid Protein Concentration, Interstitial Fluid Volume, and Interstitial Fluid Pressure – lymphatic system controls concentration of proteins in interstitial fluids, volume of interstitial fluid, and interstitial fluid pressure -small amounts of proteins leak out of blood capillaries into the interstitium, with small amounts returning on the venous end -proteins accumulate in interstitial fluid to increase colloid osmotic pressure of interstitial fluids -increasing colloid osmotic pressure in interstitial fluid shifts balance of forces at blood capillary membrane in favor of fluid filtration into interstitium -fluid is translocated osmotically outward through capillary wall by proteins and into interstitium to increase both interstitial fluid volume and pressure -increasing interstitial fluid pressure greatly increases rate of lymph flow to carry away excess volume and excess protein that has accumulated -once interstitial fluid protein concentration reaches a certain level and increases fluid volume and interstitial fluid pressure, the return of protein and fluid through lymphatics becomes great enough to balance out rate of leakage Significance of Negative Interstitial Fluid Pressure as a Means for Holding Body Tissues Together – traditionally it was thought that tissues were held together only by connective tissue fibers; but many places in the body don’t have these fibers, such as where skin slides over back of hand or face; but these tissues are still held together by NEGATIVE INTERSTITIAL PRESSURE, which is a partial vacuum -when tissues lose their negative pressure, fluid accumulates in spaces and edema occurs