Supplementary Material and Methods
advertisement

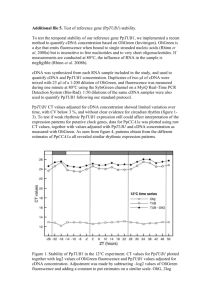
The cockroach Blattella germanica obtains nitrogen from uric acid through a metabolic pathway shared with its bacterial endosymbiont. Rafael Patiño-Navarrete1, MariaDolors Piulachs2, Xavier Belles2, Andrés Moya1, Amparo Latorre1* and Juli Peretó1,3* 1 InstitutCavanilles de Biodiversitat i Biologia Evolutiva (Universitat de València), C/ Catedràtic José Beltrán nº 2, 46980 Paterna-Spain. 2 Institut de Biologia Evolutiva (Consejo Superior de Investigaciones Científicas and Universitat Pompeu Fabra), Passeig Marítim de la Barceloneta nº 37-49, 08003 BarcelonaSpain. 3 Departament de Bioquímica i Biologia Molecular, Universitat de València. Electronic Supplementary Material Supplementary Materials and Methods Transcriptome sequencing Blattella germanica specimens used for the whole transcriptome analysis were obtained from a population reared at the Institut de Biologia Evolutiva (CSIC-UPF). Conditions were 70% humidity at 30 ºC and a commercial dog food diet. The whole transcriptome was analysed in three different tissues, fat body and ovary, which harbour Blattabacterium, although in the ovary the endosymbionts are only in transit to infect the embryos, and epidermis, which is a Blattabacterium-free tissue. Fat body and ovaries were obtained from six adult females, taken from three to five days after moulting to the adult form, while the epidermis was obtained from the thoracic dorsum of 5 to 6-day-old 6th instar nymphs. Three animals were used to pool ovary and epithelium, and five were necessary to pool the fat body. Total RNA was extracted with the GenEluteTM Mammalian Total RNA Miniprep Kit (Sigma-Aldrich). RNA samples were sent to be sequenced at GATC-Biotech (Konstanz, Germany), cDNA was synthesized using the Smart cDNA Construction Kit (Clontech). The first strand was synthesized using an oligo (dT) followed by a cap-primed second strand synthesis. The trimmed sequences were cleaned for Blattabacterium by mapping all the reads against the genome sequence of Blattabacterium str. Bge (Accession numbers NC_013454, NC_015679), using MEGABLAST (1), with an e-value cut-off of 10-5 and an identity percentage of 95. Assembly and annotation The assembly was performed using MIRA 3.2 with the options ‘denovo, est, accurate, 454 454_SETTINGS -LR:mxti=1 -ED:acc=1 -DP:ure=1’ in MIRA 3.2(2). Finally, the contigs obtained were annotated with the Blast2GO suite (3) Experimental diets The experimental diets were based on those proposed by Mullins and Cochran (4), with some modifications. Table S1 describes the composition. Adult females were separated from the colony as soon as they moulted to the adult form, before changing colour. From this moment, they were fed for 48 h with the control diet, thereafter they were placed on the experimental diet for another 48 h. On the fourth day after moulting to the adult form, fat body and ovaries of each animal were removed. After RNA extraction and cDNA synthesis, the relative expression of all genes encoding for uricolytic enzymes, glutamine synthetase and urease were measured with Real-Time-qPCR (RT-qPCR). Three biological samples were measured for each experiment, and three technical replicates were done for each sample. As a reference for all genes encoded by B. germanica the actin 5c gene (accession no. AJ862721) was used, while the gene encoding for the Blattabacterium elongation factor EF-TU (accession no. YP_00328380) as a reference to measure the relative expression of the urease gene. The results are shown graphically as copies of target mRNA per copies of a reference gene per 1000, based on the comparative CT method (5). Data were expressed as mean standard error of the mean (sem) of the biological samples. Statistical analyses between groups were tested by the REST 2008 program (Relative Expression Software Tool v 2.0.7, Corbett Research), that makes no assumptions about the distributions, evaluating the significance of the derived results by Pair-Wise Fixed Reallocation Randomization Test tool in REST (6). cDNA synthesis for RT-qPCR For cDNA synthesis, 400 ng of RNA were used. Prior to cDNA synthesis, samples were treated for 30 min at 30 ºC with one U of DNaseI (Promega). The transcriptor First Strand cDNA Synthesis Kit (Roche) was selected for cDNA synthesis. In this case, random hexamers were used as a priming method, allowing cDNA synthesis from bacterial mRNA. The whole procedure was performed according to the manufacturer's instructions, but an additional denaturation step (10 min at 65 ºC) was applied to the RNA-random hexamers mixtures to denature mRNA secondary structures that may impair hexamers binding to the template. RT-qPCR All reactions were carried out in a Lightcycler 2.0 (Roche) with the kit FluoCycle IITM SYBR® (Euroclone). Each PCR was run with 1 U of Taq polymerase and 250 mM of each primer (see primer sequences in Table S2). Temperature profile was 95 ºC for 10 min followed by 40 cycles of 95 ºC for 5 s, 55 ºC for 8 s, and 72 ºC for 20 s. Finally a melting curve analysis was performed to ensure that only one product was present. Supplementary Tables Table S1. Protein content and composition of the cockroach diets. Protein content Composition Dextrin Low protein High protein Control 0% 5% 50% ~25% 4g WSMa 4g WSMa 4g WSMa 96g dextrin 10g YEb 10g YEb 20g cellulose 106g cellulose 45g casein Commercial dog food 41g dextrin a Wesson salt mixture (7). Yeast Extract (Scharlau). b Table S2. Genes and primers used in dietary protein level experiments. Gene Primer Sequence Actin 95 96 512 URTOf URTOr ALNf ALNr ALTf ALTr BgGSaf BgGSar TU1f TU1r UC1f UC1r 5’-TCGTTCGTGACATCAAGGAGAAGC-3’ 5’-TGTCGGCAATTCCAGGGTACATGGT-3’ 5’-AGCTTCCTGATCGTCAGGTGA-3’ 5’-AAATCATTGGTGGGCTTCGTGGTC-3’ 5’-TCACGTCTGGCAACGTTCTGTACT-3’ 5’-TCTGACAGCAGAAACCTGTCACCA-3’ 5’-GCTGCCCAAAGACGTTCCTTGTTT-3’ 5’-GGAATTATGCACCTCGCTTCTCTC-3’ 5’-CACTCCCTATTCTACTGTTCCGAC-3’ 5’-TACAAAGATCCATTCAGGCCA-3’ 5’-CACGTATGCCTTTGATTTGTGG-3’ 5’-AAGGAAGAAGGAGGACGACACACT-3’ 5’-TAGGCTGATGCAATTCCACCTCCA-3’ 5’-GTCCAGCAACTGGAACTATAGCCA-3’ 5’-CCTCCTGCACCTGCTTCTATTTGT-3’ Urate oxidase Allantoinase Allantoicase Glutamine synthetase Elongation factor EF-Tu Urease Table S3. Accession numbers in European Nucleotide Archive. The E-value and the species for the best hit of each sequence after a Blastx against the NCBI is indicated. Gene Urate oxidase Allantoinase Allantoicase Aspartate aminotransferase Mitochondrial like Cytoplasmic Asparagine synthetase Glutamate dehydrogenase, mitochondrial Glutamine synthetase 2 Glutamate-semialdehyde dehydrogenase Ornithine-δ-transaminase Pyrroline-5-carboxylate reductase Isozyme P5CR Isozyme P5CR2 Phophoglycerate dehydrogenase Phosphoserine transaminase Phosphoserine phosphatase Serine hydroxymethyl transferase Accession number HG965798 HG965799 HG965800 E-value (Species) 6e-145 (Solenopsis invicta) 0.0 (Zootermopsis nevadensis) 2e-38 (Bombyx mori) HG965785 HG965786 HG965787 HG965788 HG965789 HG965801 HG965792 0.0 (Zootermopsis nevadensis) 2e-160 (Zootermopsis nevadensis) 0.0 (Zootermopsis nevadensis) 0.0 (Zootermopsis nevadensis) 0.0 (Zootermopsis nevadensis) 8e-61 (Papilio xuthus) 0.0 (Musca domestica) HG965795 HG965796 HG965790 HG965794 HG965793 HG965791 2e-88 (Zootermopsis nevadensis) 6e-44 (Tribolium castaneum) 2e-104 (Zootermopsis nevadensis) 0.0 (Zootermopsis nevadensis) 1e-54 (Locusta migratoria) 0.0 (Culex quinquefasciatus) Supplementary References 1. Altschul S, Madden T, Schäffer A, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25 ,3389–3402. (doi: 10.1093/nar/25.17.3389). 2. Chevreux B, Wetter T, Suhai S. 1999 Genome sequence assembly using trace signals and additional sequence information. Computer science and biology: proceedings of the german conference on bioinformatics (GCB). Heidelberg, Germany. pp. 45–46. 3. Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics21, 3674–3676. (doi: 10.1093/bioinformatics/bti610). 4. Mullins DE, Cochran DG. 1974 Nitrogen metabolism in the american cockroach: an examination of whole body and fat body regulation of cations in response to nitrogen balance. J. Exp. Biol. 61, 557–570. 5. Schmittgen TD, Livak KJ. 2008 Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. (doi:10.1038/nprot.2008.73). 6. Pfaffl MW, Horgan GW, Dempfle L. 2002 Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in realtime PCR. Nucleic Acids Res. 30, e36 (doi: 10.1093/nar/30.9.e36). 7. Wesson LG. 1932 A modification of the osbornemendel salt mixture containing only inorganic constituents. Science75, 339–340. (doi: 10.1126/science.75.1943.339).