Conflict of Interest (COI) Forms
advertisement

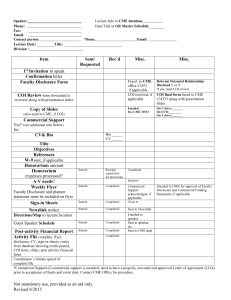
Dartmouth-Hitchcock (D-H) and the Geisel School of Medicine at Dartmouth (Geisel) Conflict of Interest (COI)/COI Resolution and Disclosure Form COI POLICY: As providers accredited by the Accreditation Council for Continuing Medical Education (ACCME) and the American Nurses' Credentialing Center's Commission on Accreditation (ANCC), Dartmouth-Hitchcock must insure balance, independence, objectivity, and scientific rigor in all its educational activities. Planning must be free of the influence or control of a commercial interest*, and promote improvements or quality in healthcare. All scientific research used to support patient care recommendations must conform to generally accepted standards of experimental design, data collection, and analysis. All Activity and Regularly Scheduled Series (RSS) Director(s), Planning Committee Member(s), Speaker(s), Author(s) or Anyone in a Position to Control the Content are expected to disclose to the audience any real or perceived conflicts of interest so that such conflicts can be resolved. COI DEFINITION: Conflict of interest is created when individuals in a position to control the content of the continuing education program, or their spouses/partners, have a relevant relationship with a commercial interest* (a) that benefits the individual in any financial amount and (b) that has occurred within the past 12 months, therefore may bias their opinions and teachings. This may include receiving a salary, royalty, intellectual property rights, consulting fee, honoraria, ownership interest (e.g. stocks, stock options or other ownership interest, excluding diversified mutual funds), or other financial benefit. Financial benefits are usually associated with roles such as employment, management position, independent contractor (including contracted research and clinical trials), consulting, speaking and teaching, membership on advisory committees or review panels, board membership, and other activities for which remuneration is received or expected. COI PROCESS: Authorized D-H and/or Geisel faculty, Deans, Directors and/or content experts will identify, review and resolve all conflicts of interest that Activity or RSS Director(s), Planning Committee Member(s), Speaker(s), Author(s) or Anyone in a Position to Control the Content disclose prior to an educational activity being delivered to learners. Please answer the following seven questions: 1) Name (Please Print): ___________________________________________________ 2) Name of Learning Activity: ______________________________________________ 3) Date(s) of Activity: ____________________________________________________ 4) Your role(s) in the learning activity (please check all that apply): Activity or RSS Director Planning Committee Member Speaker Author Other Person in a Position to Control Content (e.g. a participant in a Case Conference) 5) You and your spouse/partner relationship with industry during the past twelve months: Do you and/or your spouse/legally recognized domestic partner have any financial interest/arrangements in any amount within the past 12 months with one or more organization(s), which could be perceived as a real or apparent conflict of interest and which would reasonably appear to influence your role as a potential activity director, planning committee member, speaker or author for any Continuing Medical Education (CME) or Continuing Nursing Education (CNE) activity? NO (If NO, please move to question 6) YES, I and/or my spouse/legally recognized domestic partner have the following financial interests, arrangements, or affiliations with the following corporate organizations: Name of Company (ies) GRANT/RESEARCH SUPPORT (External) CONSULTANT MAJOR STOCKHOLDER SPEAKERS BUREAU (Specify therapeutic area) OTHER FINANCIAL INTEREST ____________________________________________________ ____________________________________________________ ____________________________________________________ ____________________________________________________ ________________________________________________ If you answered YES to Question 5, please forward a copy of your presentation materials no later than one month before the activity so that arrangements can be made for a content expert to review and resolve any potential conflict in advance of the activity. If you are a participant in a case conference (and therefore have no presentation materials), the resolution of any disclosed relationship with industry will be resolved in advance of the case conference by the RSS Director and disclosed. 6) 7) I attest that I am not receiving direct payments from a commercial entity. For CME activities only: Speakers and Participants, please answer the following questions regarding validation of clinical content of your presentation YES NO All the recommendations involving clinical medicine are based on evidence that is accepted within the profession of medicine as adequate justification for their indications and contraindications in the care of patients. YES NO All scientific research referred to, reported or used in support or justification of a patient care recommendation conforms to the generally accepted standards of experimental design, data collection and analysis. Signature______________________________ Printed Name______________________________ Date______________________ To submit electronically please type your name, save and attach to email and send to learning activity planner. *A commercial interest is any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients. This page to be completed and signed by authorized person (CME Associate Dean, CME Director, CNE Director, appointed Geisel faculty, or D-H Nurse Planner) resolving a disclosed relationship with industry on Page 1, Question 5 of this document. Dartmouth-Hitchcock (D-H) and the Geisel School of Medicine at Dartmouth (Geisel) Conflict of Interest (COI)/COI Resolution and Disclosure Form For Activity or Regularly Scheduled Series (RSS) Director(s), Planning Committee Member(s), Speaker(s), Author(s) or Anyone in a Position to Control Content Offered During an Educational Activity COI RESOLUTION PROCEDURE: If Question 5 on page 1 of the Conflict of Interest (COI)/COI Disclosure Form is answered Yes, then an authorized person (see above) must resolve the conflict of interest by taking at least one of the step(s) indicated below. The authorized person must return this form with his/her signature to the DHMC CME/CNE Office no later than one month before the activity. Planning Committee Members must disclose and be resolved or disqualified and replaced at the start of the planning process. I have resolved potential Conflict of Interest by taking one or more of the following actions (please check): 1) altering the financial relationship(s) with the commercial interest(s); Individuals may change their relationships with commercial interest (e.g. discontinue contracted services). This way, no duty, loyalty or incentive remains to introduce bias into content. 2) altering the individual’s control over content about the products or services of the commercial interest; Choosing someone else to control that part of the content. If a proposed speaker has a conflict of interest related to the content, choose someone else who does not have a relationship to the commercial interests related to the content. Change the focus of the activity. The provider could change the focus of the activity so that the content is not about products or services of the commercial interest that is the basis of the conflict of interest. Change the content of the person’s assignment. The role of a person with a conflict of interest can be changed within the activity so that it is no longer about products or services of the commercial interest. For example, an individual with a conflict of interest regarding products for treatment of a condition could address the pathophysiology or diagnosis of the condition, rather than therapeutics. Limit the content to a report without recommendations. If an individual has been funded by a commercial company to perform research, the individual’s presentation may be limited to the data and results of the research. Someone else can be assigned to address broader implications and recommendations. Limit the sources for recommendations. Rather than having a person with a conflict of interest present personal recommendations or personally select the evidence to be presented, limit the role of the person to reporting recommendations based on formal structured reviews of the literature with the inclusion and exclusion criteria stated (evidence-based). For example, the individual could present summaries from the systematic reviews of the Cochrane Collaboration. 3) validating the activity content through independent peer review; This is to ensure that all scientific research referred to, reported or used in the presentation in support or justification of patient care recommendations conforms to the generally accepted standards of experimental design, data collection and analysis. 4) disqualifying the planning committee member, speaker, author or others in a position to control content and select a replacement. Individuals who refuse to disclose relevant financial relationships will be disqualified from the development, management, presentation or evaluation of activities. EVALUATION: Activities will be evaluated by participants and peer reviewers to determine if the content was free of commercial bias and met acceptable scientific standards. For CME Associate Dean, CME Director, CNE Director, or appointed Geisel Faculty signature only: Conflicts of Interest were identified, resolved and will be disclosed to CME/CNE activity participants. Authorized Signature______________________________ Printed Name______________________________ Date_________________ 3/16/06 Approved by the DHMC/Geisel Faculty CME Advisory Committee and Nursing Continuing Education Council, Revised 12/08, 9/09, 11/10, 9/12, 11/12, 11/13, 9/14, 7/15