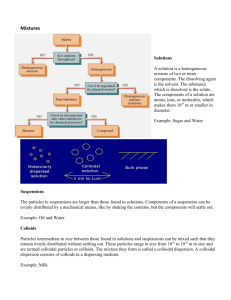
Physical and Colloidal Chemistry
advertisement

MINISTRY OF SCIENCE AND EDUCATION OF THE REPUBLIC OF KAZAKHSTAN STATE UNIVERSITY of SEMEY named after SHAKARIM Document of SQM EMCD of 3rd level EMCD 042-18-34.1.106/03-2014 EMCD The of discipline of «Physical and Edition № 1from Colloidal Chemistry» «_11_»___09___2014 EDUCATIONAL-METHODOLOGICAL COMPLEX OF DISCIPLINE on «Physical and Colloidal Chemistry» for speciality 5B011200– «Chemistry» EDUCATIONAL METHODOLOGICAL MATERIALS Semey 2014 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page2from46 Preface 1 WORKED OUT Compiled by: ______________ B.B. Bayakhmetova, Ph.D. in Chemistry, senior teacher of the Chemistry and Geography Department 2 DISCUSSED 2.1 At the meeting of Chemistry Department Protocol _1__, September _2__, 2014 Head of the Department ____________ D.R. Ontagarova 2.2 At the meeting of Educational-Methodological Bureau of the Natural Science Department Protocol _1__, September _3__, 2014 Head of the Bureau____________ Z.V. Abdisheva 3 APPROVED Approved and recommended for publishing at the meeting of the Teaching-Methodological Council of the University Protocol _1__, September _11__, 2014 Head of the TMC, Vice-principal__________________ G.К. Iskakova 4 INTRODUCED instead of edition №1 from 18.09.2013 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 Content 1 A briefsynopsis of the lectures 2 Laboratory works 3 Independentworkofstudents page3from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page4from46 The glossary on discipline " Physical and Colloidal Chemistry" Absorption is a phenomenon that occurs when matter crosses from one phase to another passing through the border surface and in the other phase more or less monotonously distributes itself in a concentration higher than the one within the first phase. Adsorption is a process in which molecules of gas, of dissolved substances in liquids, or of liquids adheres in an extremely thin layer to surfaces of solid bodies with which they are in contact. Aerosols are colloidal dispersions of liquid or solid particles in a gas, as in a mist or smoke. The commonly used aerosol sprays contain an inert propellant liquefied under pressure. The pressure of the gas causes the mixture to be released as a fine spray (aerosol) or foam (aerogel) when a valve is opened. Absorption coefficient (a) is the relative decrease in the intensity of a collimated beam of electromagnetic radiation, as a result of absorption by a medium, during traversal of an infinitesimal layer of the medium, divided by the length traversed. Adsorbent is a substance on the surface of which a substance is adsorbed. Binary solution is a mixture of two liquids that are completely miscible one with another. Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m- 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope. Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions: Dialysis a very slow process, where the aim is to remove a large part ot any ionic material that may have accompanied their formation. Sols - dispersions of small solid particles in a liquid. Emulsions are colloidal systems in which the dispersed and continuous phases are both liquids. Gels are colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material. Foams are dispersions of gases in liquids or solids. Colloid ions emerge when colloid particles adsorb certain type of ion from solution and thus become charged with the same charge. The charge can also originate form a chemical reaction of colloid particle’s surface. Colloid ions formed by absorption of silver chloride particle can be show as follows: (nAgCl)Cl- i (nAgCl)Ag+ Adsorbed layer is monomolecular (one molecule thick) and which type of ion will be formed depends upon which ions are present in a greater number in the solution in. Because of this colloid particles are charged with the same charge, mutual repelling occurs, and the colloid solution becomes stable. Colloid charge can be determined by electrophoresis. Colloid mills are machines used to grind aggregates into very fine particles or to apply very high shearing within a fluid to produce colloid suspensions or emulsions in which the particle sizes are less than 1 micrometer. One type of colloid mill is called a disc mill, in which a mixture of a solid and liquid (or two liquids) is passed between two discs a small distance apart, which rotate very rapidly relative to each other. Applications of colloid mills occur in food processing, in paint manufacture, and in the pharmaceutical industry. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page5from46 Colloid silver is a bright blue-green powder which dissolved in water gives colloid solution of red colour. Colorimeter is an instrument used to measure the strength of colorification in a solution. Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws. Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated. Flotation is a procedure in which hydrophobic solid substances are separated from hydrophilic one using bubbles of air. If air is blow through a suspension, in which substances promoting easier creation of foam are added, bubbles of air are created which stick to the hydrophobic matter and carry it out to the surface. Molar absorption coefficient (ε) is the absorption coefficient divided by amount-of-substance concentration of the absorbing material in the sample solution (ε = a/c). The SI unit is m2mol-1. Also called extinction coefficient, but usually in units of dm3cm-1mol-1. Nonpolar solvent is a liquid with nonpolar molecules. It dissolves covalent compounds, nonwater solvent. Osmotic pressure (Π) is the excess pressure necessary to maintain osmotic equilibrium between a solution and a pure solvent separated by a membrane permeable only to the solvent. In an ideal dilute solution Π = cB RT where cB is the amount-of-substance concentration of the solute, R is the molar gas constant, and T the temperature. Ostwald’s viscometer is a simple appliance used for determining relative viscosity. Phase is a portion of a physical system (solid, liquid, gas) that is homogeneous throughout, has definable boundaries, and can be separated physically from other phases. Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids. Spectrophotometry is a determination of the concentration of a material in a sample by measurement of the amount of light the sample absorbs. Thermostat is a device which controls the heating or cooling of a substance, by turning the machinery on or off, in order to maintain a constant temperature. Tyndall’s effect occurs when light disperses on colloid particles. This phenomenon can be seen when a ray of light enters in dark room through a small hole. In the beam some dust particles of colloid dimensions can be seen sparkling. Viscosity. (η) (coefficient of viscosity) is the resistance a liquid exhibits to flow. Experimentally, the frictional force between two liquid layers moving past each other is proportional to the area of the layers and the difference in flow speed between them. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page6from46 Electrostatic stabilization is based on the mutual repulsion of like electrical charges. In general, different phases have different charge affinities, so that an electrical double layer forms at any interface. Steric stabilization consists in covering the particles in polymers which prevents the particle to get close in the range of attractive forces. Spectroscopy: The study of the interaction of matter and electromagnetic radiation, usually as a function of the radiation wavelength. Calibration Calibration is a process in which the operation of the mass spectrometer in a specified manner is adjusted and certified to produce the accurate and known ion masses in the spectrum of a standard compound. Ionization energy The ionization energy is the minimum energy required to remove an electron from an atom or molecule in order to produce a positive ion. 1 LECTURES A brief synopsis of the lectures Lecture #1,2. Introduction to physical and colloid chemistry. Purpose: To familiarize with the basic group of dispersion systems Key questions: 1. The nature and classification of colloid system. 2. The basic types of dispersion systems Summary: Colloidal system is a highly dispersed system in which dispersed particles are not molecules but aggregates of many molecules. The size range consume the nanometer (10–9m) to micrometer (10–6m) range. There is no sharp distinction between colloidal and non-colloidal systems. Usually colloidal system is found due to the nature of the substances dissolved in the media and does not depend on aggregation, chemical nature and origin. Colloid science is a part of surface science. The surface interfacial phenomena associated with colloidal systems such as emulsions and forms are often studied by means of experiments on artificially flat surfaces rather than on the colloidal systems themselves. A mixture in which one substance is divided into minute particles (called colloidal particles) and dispersed throughout a second substance. The substances are present as larger particles than those found in solution, but are too small to be seen with a microscope. There are no strict boundaries on the size of colloidal particles, but they tend to vary between 10-9 m to 10-6 m in size. The mixture is also called a colloidal solution, colloidal system, or colloidal dispersion. The three forms in which all matter exists are solid, liquid or gas. Colloidal systems can be any combination of these states. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page7from46 A colloidal system is not a true solution but it is not a suspension either because it does not settle out like a suspension will over time. Colloids are larger than most inorganic molecules and remain suspended indefinitely. They are large molecules, such as proteins, or groups of molecules. They have many properties, depending on their large specific surface. Colloid formation can be classified in two systems, namely reversible and irreversible. In an irreversible system, the products are so stable or removed so well that the original reactants cannot be reproduced. A reversible system is one in which the products can be made to react to reproduce the original reactants. The word "Colloid" was derived from the Greek, "kolla" for glue, as some of the original organic colloidal solutions were glues. This term was first coined in 1862 to distinguish colloids from crystalloids such as sugar and salt. Colloids have been studied by scientists since the early 1800's. The early part of the 20 th century saw a number of major developments in both chemistry and physics, some of which had direct influences on the study of colloids. A number of methods for studying colloidal particles were developed, including diffusion, electrophoresis, and scattering of visible light and X-rays. Due to colloidal particles being so small, their individual motion changes continually as a result of random collision with the molecules of the dispersion medium. This random, zig-zagging movement is called Brownian motion after the man who discovered it. This motion helps keep the partilces in suspension. In the early 19th century, Michael Faraday showed that when you pass a strong beam of light through a colloidal solution, it is scattered. This method to study colloids was further developed by John Tyndall and became known as the "Tyndall effect". Types of colloids: Colloids are usually classified according to the original states of their constituent parts:classified according to the original states of their constituent parts: Dispersing medium Dispersed phase Name Solid Solid Solid sol Solid Liquid Gel Solid Gas Solid foam Liquid Solid Sol Liquid Liquid Emulsion Liquid Gas Foam EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page8from46 Gas Solid Solid aerosol Gas Liquid Aerosol Dispersing medium (external phase) - the constituent found in the greater extent in the colloid. Dispersed phase (internal phase) - the constituent found in the lesser extent. A further classification is as lyophilic (solvent attracting), lyophobic (solvent repelling) or association colloids (a mixture of the two). If water is the dispersing medium, it is often known as a hydrosol. Colloidal dispersions may be lyophobic (water hating) or lyophilic (water loving). Lyophilic sols are formed spontaneously when the dry coherent material (e.g. gelatin, rubber, soap) is brought in contact with the dispersion medium, hence they are thermodynamically more stable than in the initial state of dry colloid material plus dispersion medium. Lyophobic sols (e.g. gold sol) cannot be formed by spontaneous dispersion in the medium. They are thermodynamically unstable with respect to separation into macroscopic phases, but they may remain for long times in a metastable state. Preparation There are two main ways of forming a colloid; reduction of larger particles to colloidal size or condensation of smaller particles, e.g. molecules, into colloidal particles. This latter generally makes use of chemical reactions such as hydrolysis or displacement. Laboratory and industrial methods make use of several techniques. This page gives a brief overview of some of these techniques, but it should be noted that there are a broad range used in practice. A method of forming an aerosol is to tear away a liquid spray with a gas jet. The process can be helped by separating the liquid into droplets with electrostatic repulsions, done by applying a charge to the liquid. Emulsions are usually prepared by vigorously shaking the two constituents together, often with the addition of an emulsifying agent, e.g. a surfactant such as soap, in order to stabilise the product formed. Semi-solid colloids, known as gels, may be formed from the cooling of lyophilic sols that contain large linear molecules and have a much greater viscosity than the solvent. Colloids are often purified by dialysis, a very slow process, where the aim is to remove a large part ot any ionic material that may have accompanied their formation. A membrane is selected that will not allow colloid particles through but will let the solvent and ions permeate through. The method relies on diffusion, osmosis and ultrafiltration. Properties Colloidal particles are generally aggregates of numerous atoms or molecules. They pass through most filter papers, but can be detected by light-scattering, sedimentation and osmosis. A EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page9from46 characteristic of colloids is absorption, as the finely divided colloidal particles have a large exposed surface area. The chemical and physical properties of inorganic colloids can be changed dramaticaly when their size is reduced to a number of nanometers. This effect is due to the increasing importance of the colloid surface. The presence of colloidal particles in a solution has only minor effects on its colligative properties (boiling, freezing point, etc.) Thixotropy is a property exhibited by certain gels. This is where a gel appears solid and maintains its own shape until it is subjected to some force or disturbance, such as shaking. It then tends to act as a sol, flowing freely. This behavior is reversible, and the sol will return to a gel if left undisturbed. Examples of thixotropic gels include certain paints, printing inks and clays. The particles of a colloid selectively absorb ions and acquire an electric charge. The existence of an electric charge on the surfaces of the colloidal particles is a source of kinetic stability for colloids. All of the particles of a given colloid are repelled by one another as they all take on the same charge. The movement of colloidal particles through a fluid under the influence of an electric field is known as electrophoresis. Examples of colloids These are just a few of the many examples of colloids, both man-made and naturally occuring. Aerosols: Man-made: Aerosol sprays, insecticide spray, smog. Natural: Fog, clouds. Solid aerosol: Natural: Smoke, dust. Foam: Man-made: Shaving lather, whipped cream. Emulsions: Man-made: Mayonnaise, cosmetic lotion, lubricants. Natural: Milk. Sols: Man-made: Paint, ink, detergents, rubber (a latex - also occur naturally). Solid foams: Man-made: Marshmallow, styrofoam, insulation, cushioning. Gels: Man-made: Butter, jelly. Solid sols: Man-made: Certain alloys. Natural: Pearl, opal. Biological macromolecules and cells may be considered to be biocolloids and many foods are also colloidal in nature. Colloids are also an important feature of the natural environment. Questions for self-control: 1. What does the colloidal chemistry study? 2. What did types of dispersion systems classify? 3. Give examples of colloids. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page10from46 References 1. Е.Д. Щукин, А.В. Пернов, Е.А. Амелина. Коллоидная химия, 3-е изд., М.: Высш.школа, 2004г., 435с. 2. Д.А. Фридрихсберг. Курс коллоидной химии, Л.: Химия, 1984, 352 с. 3. Ю.Г. Фролов. Курс коллоидной химии: Поверхностные явления и дисперсные системы. М.: Химия, 1988, 462 с. Lecture #3, 4. Molecular – kinetic and rheological properties of colloid systems Purpose: learn the molecular – kinetic and rheological properties of colloid systems Key questions: 1. Thermal molecular motion and Brownian motion. 2. Diffusion in solutions, Osmotic pressure 3. Dispersions, sedimentation stability. Summary: Kinetic properties of colloidal systems relate to the motion of particles with respect to the dispersion medium. They are; Brownian Motion Brownian motion is seen in particular of sizes 6 mm, as a result of the bombardment of the particles by the molecules of the dispersion medium. It is not observable due to small size. The velocity of the particle increases with the decrease in particle size. Brownian movement can be stopped by increasing the viscosity of medium by the addition of glycerine or similar agents. Brownian movement: Robert Brown (1927) an English Botanist, observed that the pollen grains in aqueous suspensions were in constant motion. Similar phenomenon was, later on, found in case of colloidal solution, when observed ultra-microscopically. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page11from46 Brownian movement. This continuous and rapid zig-zag motion of the colloidal particles is called Brownian movement. This motion is independent of the nature of the colloidal particles. It is more rapid when the size of the particles is small and the solution is less viscous. Diffusion. Diffusion is another kinetic property of colloid which occur as a result of brownian movement. Here particles get diffused spontaneously from a region of higher concentration to one of lower concentration until the concentration of system is uniform throughout. According to fick's 1st law, the amount dm of a substance diffusing in time dt across an area S is directly proportional to charge of concentration dc with distance dx: dm D dc sd dx D- is the diffusion coefficient Diffusion coefficient may be obtained in colloidal chemistry by diffusion experiments in which the material is allowed to pass through a porous disc and samples are removed and analysed periodically. If the colloidal particles are assumed to be spherical, the following equation is used to obtain radius of the particle and molecular weight. RT 1 kT D N A B 6r В = 6πηr for spherical particles R = Molar gas constant T =Absolute temperature η= Visocity of the solvent NA = Avagadros number r =radius of the particles Osmotic pressure If two solutions of different concentration are separated by a semi-permeable membrane which is permeable to to the smaller solvent molecules but not to the larger solute molecules, then the solvent will tend to diffuse across the membrane from the less concentrated to the more concentrated solution. This process is called osmosis. The energy which drives the process is usually discussed in terms of osmotic pressure. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page12from46 The osmotic pressure π, is given by van't Hoff formula, which is identical to the pressure formula of an ideal gas: π = cRT where c is the molar concentration of the solute, R = 0.082 (liter∙bar) / (deg∙mol), is the gas constant, and T is the temperature on the absolute temperature scale (Kelvin). Van't Hoff received the first Nobel prize in chemistry, in 1901, for his interpretation of osmosis. Sedimentation The velocity U of sedimentation of spherical particles having density ρ in a medium of density ρ0 and η viscosity ho is given by Stoke's law. 2r 2 0 g u 9 r- radius of the particles g- velocity of acceleration if the particles are subjected only to force of gravity then the lower size limit of particles obeying stoke's equation is about 0.5 mm. this is because Brownian movement become significant and tends to offset sedimentation due to gravity and promotes mixing. 50 a stronger force must be applied to bring about the sedimentation of colloidal particles. This is accomplished by ultra centrifuge, developed by Svedberg. The instantaneous velocity U = dx/dt of a particle in a unit centrifugal field is expressed in terms of the Svedberg sedimentation coefficient, S m S B 2 2 r 0 For of spherical particles S 9 Molecular weight can be determined by two method, 1) Sedimentation velocity technique 2) Sedimentation equilibrium method VISCOSITY Viscosity is an expression of the resistance to flow of a system under an applied stress. The more viscous a liquid, the greater the applied force required to make it flow at particular rate. Viscosity studies also provide information regarding the shape of the particle in solution. Einstein developed an equation of flow applicable to dilute colloidal dispersion of spherical particles namely, h = h0 (1 + 2.5 q) h0 = viscosity of the dispersion medium h = viscosity of the dispersion when the volume fraction of colloidal particles present is q. The volume fraction is defined the volume of particles divided by the total volume of dispersion, it is therefore equivalent to a concentration term. Several viscosity coefficients may be defined with respect to this equation. These include relative viscosity, specific viscosity, intrinsic viscosity. Several viscosity coefficients may be defined with respect to this equation. These include relative viscosity, specific viscosity, intrinsic viscosity. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page13from46 Since volume fraction is directly related to concentration. in which C is expressed in grams of colloidal particles per 100 ml of total dispersion. For highly polymeric materials dispersed in the medium at moderate concentration. If is plotted against C, the line which is polated to infinite dilution, the intercept is K1 known as intrinsic viscosity (h) is used to calculate the approximate molecular weight of polymers. According to the Mark-Houwink equation, [h] = KMa K & a are constants, characteristics of the particular polymer-solvent system. M=molecular weight The characteristics of polymers used as substitutes for blood plasma depend in part on molecular weight of the material. These characteristics include the size and shape of macromolecules and the ability of the polymers to impart the proper viscosity and osmotic pressure to the blood. These methods are used to determine the average molecular weight of hydroxyl ethyl starch and gelatine preparation used as plasma extenders. Questions for self-control: 1. What is thermal molecular motion and Brownian motion? 2. What is diffusion in solutions and Osmotic pressure? 3. What is determined sedimentation velocity? References 1. Е.Д. Щукин, А.В. Пернов, Е.А. Амелина. Коллоидная химия, 3-е изд., М.: Высш.школа, 2004г., 435с. 2. Д.А. Фридрихсберг. Курс коллоидной химии, Л.: Химия, 1984, 352 с. 3. Ю.Г. Фролов. Курс коллоидной химии: Поверхностные явления и дисперсные системы. М.: Химия, 1988, 462 с. Lecture #5,6. Optical properties of colloid systems Goal: to learn the basics of optical phenomena Key questions: 1. Introduction to optical phenomena. 2. The Rayleigh’s and Bouguer-Lambert - Beer’s equations Summary: The light scattering methods for studying colloidal systems can be classified in two wide groups: SLS and DLS. The latter is often called quasi-elastic light scattering (QELS) or photon correlation spectroscopy (PCS). In SLS methods, the averagedover-time intensity of the scattered Edition № 2from “11”__09__2014 EMCD 042-18-34.1.106/03-2014 page14from46 light is measured as a function of the particle concentration and scattering angle. In DLS methods, the time fluctuations of the scattered light are measured. The light scattering methods possess a number of advantages, which make them particularly suitable for investigation of colloid systems. In general, these methods are noninvasive; applicable to very small and unstable (when dried) particles, such as surfactant micelles and lipid vesicles; suitable for characterization of particle size and shape, as well as of interparticle interactions; and relatively fast, and not requiring very expensive equipment. The law states that there is a logarithmic dependence between the transmission (or transmissivity), T, of light through a substance and the product of the absorption coefficient of the substance, α, and the distance the light travels through the material (i.e., the path length), ℓ. The absorption coefficient can, in turn, be written as a product of either a molar absorptivity (extinction coefficient) of the absorber, ε, and the molar concentration c of absorbing species in the material, or an absorption cross section, σ, and the (number) density N' of absorbers. For liquids,these relations are usually written as: whereas for gases, and in particular among physicists and for spectroscopy and spectrophotometry, they are normally written where 0 and are the intensity (or power) of the incident light and the transmitted light, respectively; σ is cross section of light absorption by a single particle and N is the density (number per unit volume) of absorbing particles. The base 10 and base e conventions must not be confused because they give different values for the absorption coefficient: . However, it is easy to convert one to the other, using The transmission (or transmissivity) is expressed in terms of an absorbance which, for liquids, is defined as whereas, for gases, it is usually defined as EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page15from46 This implies that the absorbance becomes linear with the concentration (or number density of absorbers) according to and for the two cases, respectively. Thus, if the path length and the molar absorptivity (or the absorption cross section) are known and the absorbance is measured, the concentration of the substance (or the number density of absorbers) can be deduced. Although several of the expressions above often are used as Beer–Lambert law, the name should strictly speaking only be associated with the latter two. The reason is that historically, the Lambert law states that absorption is proportional to the light path length, whereas the Beer law states that absorption is proportional to the concentration of absorbing species in the material.[1] If the concentration is expressed as a mole fraction i.e., a dimensionless fraction, the molar absorptivity (ε) takes the same dimension as the absorption coefficient, i.e., reciprocal length (e.g., m−1). However, if the concentration is expressed in moles per unit volume, the molar absorptivity (ε) is used in L·mol−1·cm−1, or sometimes in converted SI units of m2·mol−1. The absorption coefficient α' is one of many ways to describe the absorption of electromagnetic waves. For the others, and their interrelationships, see the article: Mathematical descriptions of opacity. For example, α' can be expressed in terms of the imaginary part of the refractive index, κ, and the wavelength of the light (in free space), λ0, according to In molecular absorption spectrometry, the absorption cross section σ is expressed in terms of a linestrength, S, and an (area-normalized) lineshape function, Φ. The frequency scale in molecular spectroscopy is often in cm−1, wherefore the lineshape function is expressed in units of 1/cm−1, which can look funny but is strictly correct. Since N is given as a number density in units of 1/cm3, the linestrength is often given in units of cm2cm−1/molecule. A typical linestrength in one of the vibrational overtone bands of smaller molecules, e.g., around 1.5 μm in CO or CO2, is around 10−23 cm2cm−1, although it can be larger for species with strong transitions, e.g., C2H2. The linestrengths of various transitions can be found in large databases, e.g., HITRAN. The lineshape function often takes a value around a few 1/cm−1, up to around 10/cm−1 under low pressure conditions, when the transition is Doppler broadened, and below this under atmospheric pressure conditions, when the transition is collision broadened. It has also become commonplace to express the linestrength in units of cm−2/atm since then the concentration is given in terms of a pressure in units of atm. A typical linestrength is then often in the order of 10−3 cm−2/atm. Under these conditions, the detectability of a given technique is often quoted in terms of ppm•m. The fact that there are two commensurate definitions of absorbance (in base 10 or e) implies that the absorbance and the absorption coefficient for the cases with gases, A' and α', are ln 10 (approximately 2.3) times as large as the corresponding values for liquids, i.e., A and α, EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page16from46 respectively. Therefore, care must be taken when interpreting data that the correct form of the law is used. The law tends to break down at very high concentrations, especially if the material is highly scattering. If the light is especially intense, nonlinear optical processes can also cause variances. The main reason, however, is the following. At high concentrations, the molecules are closer to each other and begin to interact with each other. This interaction will change several properties of the molecule, and thus will change the molar aborbtivity. If the absorbtivity is different at higher concentrations than at lower ones, then the plot of the absorbance will not be linear, as is suggested by the equation, so you can only use it when all the concentrations you are working with are low enough that the absorbtivity is the same for all of them. Derivation Classically, the Beer-Lambert law was first devised independently where Lambert's law stated that absorbance is directly proportional to the thickness of the sample, and Beer's law stated that absorbance is proportional to the concentration of the sample. The modern derivation of the BeerLambert law combines the two laws and correlate the absorbance to both, the concentration as well as the thickness (path length) of the sample. In concept, the derivation of the Beer–Lambert law is straightforward. Divide the absorbing sample into thin slices that are perpendicular to the beam of light. The light that emerges from a slice is slightly less intense than the light that entered because some of the photons have run into molecules in the sample and did not make it to the other side. For most cases where measurements of absorption are needed, a vast majority of the light entering the slice leaves without being absorbed. Because the physical description of the problem is in terms of differences—intensity before and after light passes through the slice—we can easily write an ordinary differential equation model for absorption. The difference in intensity due to the slice of absorbing material is reduced; leaving the slice, it is a fraction of the light entering the slice . The thickness of the slice is , which scales the amount of absorption (thin slice does not absorb much light but a thick slice absorbs a lot). In symbols, , or . This conceptual overview uses to describe how much light is absorbed. All we can say about the value of this constant is that it will be different for each material. Also, its values should be constrained between −1 and 0. The following paragraphs cover the meaning of this constant and the whole derivation in much greater detail. Assume that particles may be described as having an absorption cross section (i.e., area), σ, perpendicular to the path of light through a solution, such that a photon of light is absorbed if it strikes the particle, and is transmitted if it does not. Define z as an axis parallel to the direction that photons of light are moving, and A and dz as the area and thickness (along the z axis) of a 3-dimensional slab of space through which light is passing. We assume that dz is sufficiently small that one particle in the slab cannot obscure another particle in the slab when viewed along the z direction. The concentration of particles in the slab is represented by N. It follows that the fraction of photons absorbed when passing through this slab is equal to the total opaque area of the particles in the slab, σAN dz, divided by the area of the slab A, which yields σN dz. Expressing the number of photons absorbed by the slab as dIz, and the total number of photons incident on the slab as Iz, the number of photons absorbed by the slab is given by Note that because there are fewer photons which pass through the slab than are incident on it, dIz is actually negative (It is proportional in magnitude to the number of photons absorbed). EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page17from46 The solution to this simple differential equation is obtained by integrating both sides to obtain Iz as a function of z The difference of intensity for a slab of real thickness ℓ is I0 at z = 0, and Il at z = ℓ. Using the previous equation, the difference in intensity can be written as, rearranging and exponentiating yields, This implies that and The derivation assumes that every absorbing particle behaves independently with respect to the light and is not affected by other particles. Error is introduced when particles are lying along the same optical path such that some particles are in the shadow of others. This occurs in highly concentrated solutions. In practice, when large absorption values are measured, dilution is required to achieve accurate results. Measurements of absorption in the range of to 1 are less affected by shadowing than other sources of random error. In this range, the ODE model developed above is a good approximation; measurements of absorption in this range are linearly related to concentration. At higher absorbances, concentrations will be underestimated due to this shadow effect unless one employs a more sophisticated model that describes the non-linear relationship between absorption and concentration EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 Questions for self-control: 1. give a classification of optical phenomena 2. what is the The Rayleigh’s equation based? 3. what is the Bouguer-Lambert - Beer’s based? page18from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page19from46 References 1. Е.Д. Щукин, А.В. Пернов, Е.А. Амелина. Коллоидная химия, 3-е изд., М.: Высш.школа, 2004г., 435с. 2. Д.А. Фридрихсберг. Курс коллоидной химии, Л.: Химия, 1984, 352 с. 3. Ю.Г. Фролов. Курс коллоидной химии: Поверхностные явления и дисперсные системы. М.: Химия, 1988, 462 с. Lecture # 7-9. Electrical properties of dispersions Purpose: To familiarize with the main types of electrokinetic phenomens Key questions: 1. Introduction to electrokinetic phenomena. 2. Structure of the electric double layer (Theories of Helmholz - Perrin, Gouy-Chapman and Stern). 3. Effects of electrolytes on zeta-potential, Electrophoresis and Electroosmosis, Measurement of zeta-potential, Isoelectric point. Summary The term ‘‘electrokinetic phenomena’’ refers to several processes which appear when a charged surface (or colloidal particle) is set in a relative motion with respect to the adjacent liquid phase. Classically, four types of electrokinetic phenomena are distinguished: electroosmosis, streaming potential, electrophoresis, and sedimentation potential Electrophoresis – a suspended, charged particle moves as a result of an applied electrical field • Sedimentation potential – an electrical potential created by the movement of charged particles through a liquid by gravity • Electrosmosis – a liquid flows along a charged surface when an electric field is applied parallel to the surface • Streaming potential – an electric potential created when a liquid is forced to move along a charged surface Electrophoresis - Movement of particle in a stationary fluid by an applied electric field. Electroosmosis - Movement of liquid past a surface by an applied electric field EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page20from46 Streaming potential - Creation of an electric field as a liquid moves past a stationary charged surface Sedimentation potential - Creation of an electric field when a charged particle moves relative to stationary fluid Electrophoresis the movement of a charged particle through a liquid under the influence of an applied potential difference (electric current) Voltage source Electroosmosis. The movement of a liquid relative to an immobile charged surface of a capillary tube under the influence of an electric field EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page21from46 Sedimentation potential (“Dorn-effect) creation of an electric field when a charged particle moves relative to stationary fluid ΔE = sedimentation potential Streaming potential creation of an electric field as a liquid moves along a stationary charged surface EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page22from46 ”steady state” conditions: ΔE = streaming potential i = flow current A solid surface in contact with a solution of an electrolyte usually carries an electric charge, σo. This gives rise to an electric potential, ψo, at the surface, and a decreasing potential, ψ, as we move through the bulk solution away from the surface, and in turn this effect the distribution of ions in the liquid. Two regions are of primary importance: the Stern layer immediately adjacent to the surface where ion size is important; and outside this region there is a diffuse layer. Because of difference in charge between the diffuse layer and the solid suface, movement of one relative to the other will cause charge separation and hence generate a potential difference, or alternatively, application of an electrical potential will cause movement of one relative to the other. The relative movement of the solid surface and the liquid occurs at a surface of shear. The potential at the shear plane is known as the zeta (ζ) potential and its value can be determined by measurement of electrokinetic phenomena. Zeta potential is almost identical with the Stern potential thus gives a measure of the potential at the beginning of the diffuse layer. EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 Electrical Double Layer Theory 1. Helmholtz model (1879) page23from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page24from46 2. Guoy Chapman Model (1910-1913) Stern Model (1924) Takes into account specifically adsorbed ions, Stern layer. Defined a slip plane between specifically adsorbed ions and diffuse layer of the electrolyte solution. Potential at this plane is called . EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page25from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page26from46 Questions for self-control: 1. 2. 3. 4. 5. 6. What types of electrokinetic phenomens do you know? What is Zeta potential? What causes the surface charge on oxides? What is the significance of and 1/ ? What does mean by "compression of electrical double layer" ? How do the three models for the electrical double layer differ from each other? References 1. Е.Д. Щукин, А.В. Пернов, Е.А. Амелина. Коллоидная химия, 3-е изд., М.: Высш.школа, 2004г., 435с. 2. Д.А. Фридрихсберг. Курс коллоидной химии, Л.: Химия, 1984, 352 с. 3. Ю.Г. Фролов. Курс коллоидной химии: Поверхностные явления и дисперсные системы. М.: Химия, 1988, 462 с. Lecture # 10- 13. Surface chemistry and phenomens Goal: to learn the basics of Surface chemistry and phenomens Key questions: 1. Structure of the surface and interface, surface tension, energy of the surface. 2. Surface active and inactive substances. 3. The types of adsorption, chemical and physical adsorption. 4. The kinetic of adsorption, influence of the properties of adsorbent and adsorptive on adsorption, adsorption equation of Gibbs. 5. Nature of adsorption forces, Langmuir monomolecular adsorption theory. 6. The polymolecular theory of Polany and BET-theory. Summary Measurement of surface tension of solutions by Du Nouy tensiometer Theory The molecules at the surface of a liquid are subjected to an unbalanced force of molecular attraction as the molecules of the liquid tend to pull those at the surface inward while the vapor does not have as strong an attraction. This unbalance causes liquids to tend to maintain the smallest surface possible. The magnitude of this force is called the surface tension.When this lowest possible energetic state is achieved the surface tension acts to hold the surface together where the force is parallel to the surface. The symbol for surface tension is "gamma". Conventionally the tension between the liquid and the atmosphere is called surface tension while the tension between one liquid and another is called interfacial tension. Edition № 2from “11”__09__2014 EMCD 042-18-34.1.106/03-2014 page27from46 The specific surface free energy or surface tension of surface γ is equal to the expenditure of work required to increase the net area of surface isothermally and reversibly way by unit of area, in 2 J m (Joule per meter); if the increase in the surface area is accomplished by moving a unit length of line segment in a direction perpendicular to itself, γ is equal to the force, or “tension”, opposing the -1 moving of the line segment. Accordingly, it is usually expressed in units of N m (Newton per meter). Surface may be a free surface (exposed to air or vapor or vacuum) or an interface with another liquid or solid. In the event that the surface is an interface, this quantity is called interface tension. The value of surface tension is dependent on the nature of the liquid and also on the temperature (see Eötvös and Ramsay empirical rule). The temperature of measurement of surface tension must be kept constant. It is found that the surface tensions of solutions are in general different from those of the corresponding pure solvents. It has also been found that solutes whose addition results in a decrease in surface tension tend to concentrate slightly in the neighborhood of the surface (positive surface concentration). The migration of solute either toward or away from the surface is always such as to make the surface tension of the solution (and thus the free energy of the system) lower than it would be if the concentration of solute were uniform throughout (surface concentration equal to zero). Equilibrium is reached when the tendency for free-energy decrease due to lowering surface tension is balanced by the opposing tendency for free-energy increase due to increasing nonuniformity of solute concentration near the surface. A surface-active molecule, also called a surface active agent or surfactant, possesses approximately an equal ratio between the polar and nonpolar portions of the molecule. When such a molecule is placed in an oil-water system, the polar group(s) are attracted to or oriented toward the water, and the nonpolar group(s) are oriented toward the oil. This orientation of amphiphilic molecules is described by Hardy-Harkins principle of continuity. The surfactant is adsorbed or oriented in this manner, consequently lowering interfacial tension between the oil and water phase. When a surfactant is placed in a water system it adsorbs at the surface and lowers the surface tension between the water and air. When it is place in a mixture of solid and liquid it adsorbs on the surface of the solid and lowers the interfacial tension between the solid and the water. Since the surfactant is adsorbed at the surface it is logical that the concentration of surfactant at the surface would be greater than the concentration in the bulk solution. Mathematically such a relationship has been derived by Willard Gibbs. It relates lowering of surface tension to excess concentration of surfactant at the surface. The Gibbs equation can write for a dilute solution in two forms: Г C d RT dC -3 where c is the concentration (in mol m ) in the solution, T (K) the absolute temperature, R the gas -1 -1 -1 2 constant (8.314 JK mol ), γ (Nm ) the surface tension and Γc (mol m ) is the surface excess concentration. It follows from Equation 1 that Γc is positive if dγ/dc is negative, that is the surface tension decreases with increasing solute concentration. On the basis of experimental surface tension vs. solute concentration function the dγ/dc can be determined and the Γc =f (c) adsorption isotherm (Eq. 2) can be calculated. Г Г bC 1 bC (2a) Edition № 2from “11”__09__2014 EMCD 042-18-34.1.106/03-2014 page28from46 The area (φm) occupied per molecule is determined as: φm=1/Γ∞ NA where NA is the Avogadro’s 23 -1 2 number (6×10 mol ). The φm for alcohols are about 0.22 nm and for carboxylic acids about 0.25 2 2 nm extrapolated from the liquid condensed regions and φm are about 0.20 nm extrapolated from the solid regions of two-dimensional isotherm of monolayer, idependent of both the length of the hydrocarbon chain and the nature of the head. Adhesion and Cohesion To understand the origin of surface tension at a molecular level one needs to look at adhesion and cohesion. Adhesion is the attraction between two different phases. Cohesion is the attraction between molecules of the same phase. We can define two quantities Work of adhesion per unit area, WAB, which is the work required to pull two liquid phases apart and measures the attraction between the two phases. A WAB A B = final -initial = A+B -AB B Work of cohesion per unit area, WAA, which is the work required to pull a liquid apart. It measures the attraction between the molecules of the liquid WAA A =2 A A A Edition № 2from “11”__09__2014 EMCD 042-18-34.1.106/03-2014 page29from46 The difference between the work of adhesion and the work of cohesion of two substances defines a quantity known as the spreading coefficient of A on B, SA/B: SA/B Physical: physisorption Chemical: chemisorption = WAB - WAA= B - A - AB= B- ( A+ AB) Physisorption Van der Waals forces between molecules multilayer adsorption predominates at low temperatures occurs rapidly reversible heat of adsorption less than 40 kJ mol-1 Chemisorption Chemical bond formed that is stronger than Van der Waals Heat of adsorption> physisorption ( > 80 kJ mol-1) irreversible activation energy involved monolayer chemical adsorption decreases at low temperatures. Adsorption from solution Theory Adsorption is the enrichment (positive adsorption, or briefly, adsorption) or depletion (negative adsorption) of one or more components in an interfacial layer. The material in the adsorbed state is called the adsorbate, while that present in one or other (or both) of the bulk phases and capable of being adsorbed may be distinguished as the adsorptive. When adsorption occurs (or may occur) at the interface between a fluid phase and a solid, the solid is usually called the adsorbent. Sorption is also used as a general term to cover both adsorption and absorption. Adsorption from liquid mixtures is said to have occurred only when there is a difference between the relative composition of the liquid in the interfacial layer and that in the adjoining bulk phase(s) and observable phenomena result from this difference. For liquids, accumulation (positive adsorption) of one or several components is generally accompanied by depletion of the other(s) in the interfacial layer; such depletion, i.e. when the equilibrium concentration of a component in the interfacial layer is smaller than the adjoining bulk liquid, is termed negative adsorption and should not be designated as desorption. Equilibrium between a bulk fluid and an interfacial layer may be EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page30from46 established with respect to neutral species or to ionic species. If the adsorption of one or several ionic species is accompanied by the simultaneous desorption (displacement) of an equivalent amount of one or more other ionic species this process is called ion exchange. It is often useful to consider the adsorbent/fluid interface as comprising two regions. The region of the liquid phase forming part of the adsorbent/liquid interface may be called the adsorption space while the portion of the adsorbent included in the interface is called the surface layer of the adsorbent. With respect to porous solids, the surface associated with pores communicating with the outside space may be called the internal surface. Because the accessibility of pores may depend on the size of the fluid molecules, the extent of the internal surface may depend on the size of the molecules comprising the fluid, and may be different for the various components of a fluid mixture (molecular sieve effect). In monolayer adsorption all the adsorbed molecules are in contact with the surface layer of the adsorbent. In multilayer adsorption the adsorption space accommodates more than one layer of molecules and not all adsorbed molecules are in contact with the surface layer of the adsorbent. The surface coverage (θ) for both monolayer and multilayer adsorption is defined as the ratio of a the amount of adsorbed substance to am the monolayer capacity (the area occupied by a molecule in a complete monolayer); a/am=θ. Micropore filling is the process in which molecules are adsorbed in the adsorption space within micropores. The micropore volume is conventionally measured by the volume of the adsorbed material, which completely fills the micropores, expressed in terms of bulk liquid at atmospheric pressure and at the temperature of measurement. Capillary condensation is said to occur when, in porous solids, multilayer adsorption from a vapour proceeds to the point at which pore spaces are filled with liquid separated from the gas phase by menisci. The concept of capillary condensation loses its sense when the dimensions of the pores are so small that the term meniscus ceases to have a physical significance. Capillary condensation is often accompanied by hysteresis. Adsorption from solution is important in many practical situations, such as those in which modification of the solid surface is of primary concern (e.g. the use of hydrophilic or lipophilic materials to realize stable dispersions in aqueous or organic medium, respectively) and those which involve the removal of unwanted material from the solution (e.g. the clarification of sugar solutions with activated charcoal). Adsorption processes are very important in chromatography, too. The theoretical treatment of adsorption from solution is general, complicated since this adsorption always involves competition between solute(s) and solvent. The degree of adsorption at a given temperature and concentration of solution depends on the nature of adsorbent, adsorbate and solvent. Adsorption from solution behaviour can often be predicted qualitatively in terms of the polar/ nonpolar nature of the solid and of the solution components. A polar adsorbent will tend to adsorb polar adsorbates strongly and non-polar adsorbates weakly, and vice versa. In addition, polar solutes will tend to be adsorbed strongly from non-polar solvents (low solubility) and weakly from polar solvents (high solubility), and vice versa. Experimentally, the investigation of adsorption from solution is comparatively simple. A known mass of adsorbent solid is shaken with a known volume of solution at a given temperature until there is no further change in the concentration of supernatant solution. This concentration can be determined by a variety of methods involving colorimetry, spectrophotometry, refractometry, surface tension, also chemical and radio-chemical methods where is appropriate. The apparent amount of solute adsorbed per mass unit of adsorbent can be determined from the change of solute concentration in the case of diluted solution. The specific adsorbed amount can be calculated as follows: EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page31from46 V С С о (1) m where or a is the specific adsorbed amount, mg/g , at constant volume , V is the volume of solution, а 3 dm , m is the mass of adsorbent, g in volume V, c0 and c are the initial and equilibrium 3 concentrations , mg/dm of dissolved substance, respectively. Subscript 1 denotes the dissolved substance (the adsorbate). In many practical cases it is found that the adsorption obeys an equation known as the Langmuir isotherm. This equation was derived for adsorption of gases on solids and assumes that: 1. the adsorption is limited to monolayer 2. and occurs on a uniform surface; i.e. all “sites” for adsorption are equivalent, 3. adsorbed molecules are localized, 4. no interaction between molecules in a given layer; independent stacks of molecules built up on the surface sites. The Langmuir equation may be written as follows: a C (2) а m 1/ b C s where a m is the monolayer capacity or the amount adsorbed at saturation, c is the equilibrium concentration of solute and b is a constant. The monolayer capacity can be estimated either directly from the actual isotherm or indirectly by applying the linear form of the Langmuir equation which is given by: C C 1 (3) a am b am A plot of c/a against c must be a linear line with a slope of 1/am The surface area of the solid (usually expressed as square meters per gram) may be obtained from the derived value of am provided that the area occupied by the adsorbed molecule on the surface is known with reasonable certainty. Asurface=amNA y (4) where NA is Avogadro’s number and y is the cross-sectional are of an adsorbed molecule. Recall that the surface area determined by this method is the total area accessible to the solute molecules. If these are large (e.g. dyestuffs, long chain molecules) they may not penetrate the pores and cracks, and the area obtained may be only a fraction of the true surface area of the solid. Adsorption Isotherms Type 1 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 Rapid rise, limiting value Monolayer Langmuir type Example of 1. Chemisorption isotherm 2. Physisorption onto a solid with very fine pore structure eg nitrogen on microporous carbon at 77K Type 2 increasing positive slope multilayer physisorption Langmuir Isotherm Assumptions page32from46 all adsorption sites equivalent Edition № 2from “11”__09__2014 EMCD 042-18-34.1.106/03-2014 page33from46 ability of adsorbate to bind is independent of whether the adjacent sites are occupied or not. adsorbate behaves as an ideal gas in gas phase only monomolecular adsorption takes place adsorbed molecules occupy fixed sites heat of adsorption is independent of surface coverage. BET Adsorption Isotherms (Brunauer, Emmett, Teller) Assumptions: 1. Multiple layers form and langmuir model applies to each layer. 2. Heat of adsorption, Hads for first layer has a value determined by properties of surface and adsorbate, but for second and all subsequent layers, it is equal to heat of vapourization H vap. 3. Evaporation (or desorption) only occurs from exposed surfaces. 4. Rate of evaporation is equal to rate of condensation on preceding layer. BET Equation v cx Vm 1 x 1 c 1 x x=p/po p is gas pressure po is vapour pressure c = constant related to heat of adsorption and desorption Gas-solid interfaces, adsorption Two types of adsorption- physisorption, chemisorption Adsorption Isotherms Langmuir type for monolayer adsorption BET type for multilayer adsorption Langmuir Equation α=bpa/ ( 1 + bpa) EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page34from46 More direct form for experimental data is pa/v = pa/Vm + 1/bV BET Equation v cx Vm 1 x 1 c 1x For plotting purposes the equation arranged as c 1x x 1 , where x p / p o V 1 x cVm cVm Gas-solid adsorption important in heterogenous catalysis To measure wetting tendency - measure contact angle If contact angle less than 90°C then liquid wets surface If contact angle greater than 90°C then liquid doesn't wet surface. Wetting - displacement from a surface of one fluid by another. Water wets a surface by displacing air Young's Equation Contact angle related to of various components. Consider a liquid making an equilibrium contact angle, , to spread an infinitesimal amount so as to cover an extra area, dA, of a solid surface. The increase in the free energy of the system is given by Edition № 2from “11”__09__2014 EMCD 042-18-34.1.106/03-2014 dG = SLdA + LG dAcos - page35from46 SGdA If the system is at equilibrium dG = 0 and SL SG + LG cos - SG = 0 (Young's Equation) surface tension of solid in equilibrium with vapour of wetting liquid. Many surfaces are modified in order to change there wetting properties Examples Fabrics treated to become water repellent Soils treated to become wetting Mineral ores and ink particles treated to make them hydrophobic so they can be separated by flotation Detergents made to wet a surface to remove oil or dirt Dips for sheep and cattle Insecticide and horticultural sprays Dry cleaning Figure 4.2 Detachment of oily dirt from a solid surface. The sequences (left to right) show: (a) the substrate/dirt system in contact with pure water, (b) the lowering of contact angle caused by detergent [ (1) < 90°C, (2) > 90°C], and (c) and (d) mechanical (hydraulic) detachment of oil droplets. Contact angle of a liquid on a solid- measure of wetting tendency Young’s Equation - relationship between contact angle and surface tensions. SG = 0 LS + LG cos - EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page36from46 Adsorption of liquids onto solids similar to gases onto solids. More complicated. Adsorption from solution involves competition between solute and solvent for adsorption sites. Physisorption. Generally follows Langmuir Isotherm. Depends on properties of solid, solute, concentration of solute, temperature. Polymers adsorb as trains, loops and tails. Conformation of polymer on surface changes with time. Gradual displacement of lower molecular weight fractions by higher molecular weight fractions.Adsorption dependent on pH, ionic strength and solvent. Polymers used for flocculating solid particles. Too much polymer adsorbed. Steric stabilisation occurs. Questions for self-control: 1. 2. 3. 4. 5. What types of Surface phenomens do you know? What is surface tension? What is the significance of Adhesion and Cohesion? What does mean by chemical and physical adsorption? How do the Langmuir monomolecular adsorption theory differ from polymolecular theory of Polany and BET-theory? Lecture # 14, 15. Stability and Coagulation of dispersions Goal: to learn the basics of Stability and Coagulation of dispersions Key questions: 1. Stability ratio and overall flocculation rate. 2. Kinetic of coagulation, interparticle energy potential, solvation, structural-mechanical and entropy effects, coagulation through electrolytes. 3. Adsorption phenomena and coagulation, Micelle. The stability of a colloidal disperse system is strongly dependent on the attractive pair potential, VA, between the dispersed particles. VA is determined by the geometric arrangement, G, of the particles (e.g. lamella-lamella; sphere-sphere; sphere-lamella; etc. interactions, independently of the chemical composition), and the Hamaker constant, A, of the overall system (which depends on the chemical composition of the constituting species, but is independent of the geometrc arrangement). Formally: VA = A × G The Hamaker constant A of the overall system originates from a combination of the individual Hamaker constants, Ai, of the dispersion medium and that of the dispersed particles. It can be EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page37from46 derived from the summation of the der Waals dispersion forces between the constituting species (dispersion medium; dispersed particles). EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page38from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page39from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page40from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page41from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page42from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page43from46 EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page44from46 A micelle (pronounced /maɪˈsɛl/ or /maɪˈsiːl/, plural micelles, micella, or micellae) is an aggregate of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the packing behavior of single-tailed lipids in a bilayer. The difficulty filling all the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal phase micelle (oil-in-water micelle). Inverse micelles have the head groups at EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page45from46 the centre with the tails extending out (water-in-oil micelle). Micelles are approximately spherical in shape. Other phases, including shapes such as ellipsoids, cylinders, and bilayers, are also possible. The shape and size of a micelle is a function of the molecular geometry of its surfactant molecules and solution conditions such as surfactant concentration, temperature, pH, and ionic strength. The process of forming micelles is known as micellisation and forms part of the Phase behaviour of many lipids according to their polymorphism. Individual surfactant molecules that are in the system but are not part of a micelle are called "monomers". Lipid micelles represent a molecular assembly, in which the individual components are thermodynamically in equilibrium with monomers of the same species in the surrounding medium. In water, the hydrophilic "heads" of surfactant molecules are always in contact with the solvent, regardless of whether the surfactants exist as monomers or as part of a micelle. However, the lipophilic "tails" of surfactant molecules have less contact with water when they are part of a micelle—this being the basis for the energetic drive for micelle formation. In a micelle, the hydrophobic tails of several surfactant molecules assemble into an oil-like core the most stable form of which has no contact with water. By contrast, surfactant monomers are surrounded by water molecules that create a "cage" of molecules connected by hydrogen bonds. This water cage is similar to a clathrate and has an ice-like crystal structure and can be characterized according to the hydrophobic effect. The extent of lipid solubility is determined by the unfavorable entropy contribution due to the ordering of the water structure according to the hydrophobic effect. Micelles composed of ionic surfactants have an electrostatic attraction to the ions that surround them in solution, the latter known as counterions. Although the closest counterions partially mask a charged micelle (by up to 90%), the effects of micelle charge affect the structure of the surrounding solvent at appreciable distances from the micelle. Ionic micelles influence many properties of the mixture, including its electrical conductivity. Adding salts to a colloid containing micelles can decrease the strength of electrostatic interactions and lead to the formation of larger ionic micelles. This is more accurately seen from the point of view of an effective charge in hydration of the system. When surfactants are present above the CMC (Critical micelle concentration), they can act as emulsifiers that will allow a compound that is normally insoluble (in the solvent being used) to dissolve. This occurs because the insoluble species can be incorporated into the micelle core, which is itself solubilized in the bulk solvent by virtue of the head groups' favorable interactions with solvent species. The most common example of this phenomenon is detergents, which clean poorly soluble lipophilic material (such as oils and waxes) that cannot be removed by water alone. Detergents also clean by lowering the surface tension of water, making it easier to remove material from a surface. The emulsifying property of surfactants is also the basis for emulsion polymerization. Micelle formation is essential for the absorption of fat-soluble vitamins and complicated lipids within the human body. Bile salts formed in the liver and secreted by the gall bladder allow micelles of fatty acids to form. This allows the absorption of complicated lipids (e.g., lecithin) and lipid soluble vitamins (A, D, E and K) within the micelle by the small intestine. Micelles are used for targeted drug delivery. 2 LABORATORY WORKS 3 INDEPENDENT WORK OF STUDENTS List of topics for independent work of students EMCD 042-18-34.1.106/03-2014 Edition № 2from “11”__09__2014 page46from46 1. The first law of thermodynamics. 2. Thermochemistry. 3. The second law of thermodynamics. 4. Entropy and its change in various processes. 5. Thermodynamic functions of the state. 6. Helmholtz energy, Gibbs energy, Gibbs-Helmholtz equations, chemical potential. 7. Thermodynamicsofchemicalequilibrium. 8. Isotherm equation, isobars and isochores of chemical reactions. 9. Rule of Gibbs phase. 10. Clapeyron- Clausius equation. 11. State diagrams of one-component systems. 12. State diagrams of two-component systems. 13. State diagrams of three-component systems. 14.Conductivity of electrolyte solutions. 15. Thermodynamics of electrode processes. 16. Effect of temperature on the rate of chemical reactions. 17. Theory of active collisions. 18. Basic concepts of the theory of the activated complex. 19. Gibbs surface energy. 20. Surface tension. 21. Adsorption at the boundary of liquid-liquid, liquid-gas. 22. Gibbs adsorption isotherm equation. 23. Adsorption at the interface between gas-solid, liquid- solid. 24. Langmuir adsorption isotherm equation. 25. Osmosis. Sedimentation. Optical properties of disperse systems. 26. The structure and electrical charge of the colloidal particle. 27. Stability and coagulation of dispersed systems. 28. Types of resistance. Sustainability factors. Coagulation of sols by electrolytes. 29. Coagulation kinetics of disperse systems. Gelation. 30. Colloidal protection. 31. Coagulation theory. 32. Classes of disperse systems. 33. Sprays, suspensions, emulsions, their properties. 34. Lyophilic disperse systems formed micelle-forming surface - active substances.