Request for Proposal
advertisement

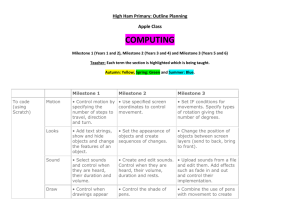
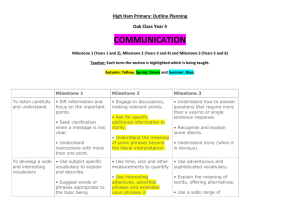
Request for Proposals WRCE American Recovery and Reinvestment Act Supplement Issue Date: April 15, 2009 Application Deadline: May 11, 2009 Approximate Award Date: June, 2009 Purpose The Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (WRCE) is eligible to receive support from the recently signed American Recovery and Reinvestment Act of 2009 (ARRA). All areas of investigation that are suitable for RCE funding (as outlined in the RFA AI-08-002; see http://grants.nih.gov/grants/guide/rfa-files/RFA-AI-08-002.html) are eligible for support with these funds, including proof-of-principle work, product-oriented studies, and basic research. It is important to remember that the primary goals of the RCEs are to develop vaccines, therapeutics, and diagnostics for NIAID Category A, B, and C priority pathogens and emerging infectious disease agents. The RCE program was initiated and funded by NIH/NIAID in September of 2003 through the U54 grant mechanism. The current 5-year grant cycle began on 3/1/09. To qualify for support through the ARRA, projects must be exceptionally innovative. The research must demonstrate that it is of high risk but has the potential for high payoff if successful. It is expected that while these “out of the box” projects entail greater than the usual degree of scientific risk, they hold the potential for delivering transformational results or paradigm shifts. While the funding for these projects will terminate no more than two years after the award, it is expected that many of them will be able to continue with support from other sources. Eligibility The Project Leader must reside within Region VI (Texas, New Mexico, Arkansas, Oklahoma, and Louisiana). Collaborators may reside outside the region. Foreign components are not allowed. Previous experience in the fields of biodefense or emerging infectious diseases is not required, and there is no requirement that the proposed research align with the thematic goals of WRCE. Proposals that were previously included in applications in response to RFA AI-08-002 are not eligible, and existing RCE projects may not be supplemented. Minorities, women, and individuals with disabilities are encouraged to apply. Award Specifics A project should request a minimum of $200,000 in direct costs per year for one or two years. All funds must be spent by the end of the award period. No clinical trials will be supported, and any involvement of human subjects must not entail more than minimal risk. Application Process Applications received after the deadline of 3pm CDT on Tuesday, May 11, 2009 will not be accepted. Please use current NIH PHS398 forms (rev. 11/07) supplied by the WRCE, available by emailing kischuen@utmb.edu. These forms have specific formatting, correct headers and footers, and section breaks, which should not be altered in any way. The forms include only the following: 1. Form Page 1: Face Page, institutional signatures not required. If the application is selected for award, we will request formal institutional signatures at that time. Dates of proposed period of support should 1 2. 3. 4. 5. 6. be one or two years starting in June 2009. If the application is selected for submission to NIAID, institutional signatures will be required at that time and no adjustments to the bottom line budget will be allowed. Form Page 2: summary, relevance, performance sites, key personnel, significant contributors, embryonic stem cells). Multiple PIs are not allowed on WRCE projects. Form Page 4: Detailed Budget for Initial Funding Period. DO NOT USE THE MODULAR BUDGET. Form Page 5: Budget for Entire Period (up to 2 years) and justification. Checklist Form Page. Biographical Sketch and Research Support for all Key Personnel. Research Plan: Up to 5 pages for sections 1-4, including tables, graphs, figures, charts, and milestones. Clearly identify why the proposed research is highly innovative and addresses major challenges. The following sections must be included, even if they are not applicable to your submission: 1. Specific Aims 2. Background and Significance 3. Preliminary Studies (not required) 4. Research Design and Methods. 4.a. Timetable and milestones for product development (see below) 5. Bibliography & References Cited 6. Protection of Human Subjects. Any project that includes any human subjects work must also include a concept sheet using a standard format; contact Rosina Cobbs for these forms. Human Subjects or Vertebrate Animal questions should be directed to Rosina Cobbs (rcobbs@utmb.edu, 409-747-0745). 7. Inclusion of Women and Minorities 8. Targeted/Planned Enrollment Table 9. Inclusion of Children 10. Vertebrate Animals 11. Select Agent Research 12. Multiple PI Plan (does not apply for RCE grants) 13. Consortium/ Contractual Arrangements. The role of each participant must be clearly indicated in Section 4. 14. Letters of Support 15. Resource Sharing Plan(s) 16. BSL3/4 Access Face Page (Form Page 1) of consortium/collaborators, if applicable. Budget Pages (Form Pages 4&5) of consortium/collaborators, if applicable. Do not use third tier subcontracts; UTMB must be the subcontracting authority. Checklist Form Page of consortium/collaborators, if applicable. Subcontracts with institutions outside the WRCE region are discouraged, unless the subcontract is absolutely required for the project and is highly justified. Submit 18 copies. Applicants are responsible for making all color copies. Submit one electronic file (either DOC or RTF; PDFs and other formats will not be accepted) by either emailing to kischuen@utmb.edu or submitting on CD with hard copies. Files over 5MB should not be emailed and must be submitted on CD. Appendices will not be accepted. Both the hard copies and the electronic files must be received no later than 3pm CDT on Tuesday, May 11, 2009. Example of timetable and milestone outline (section 4.a.) The applicant must submit a detailed milestone chart (hard copy and electronic) that includes specific aims. An example is given below. Gantt charts, Excel spreadsheets, or Word tables are acceptable. Specific aims are considered too broad to be individual milestones. Product development activities should be included, as applicable. 2 Task # Task Name Start Date End Date 1 Specific Aim #1: To develop an attenuated strain of virus X for vaccine purposes. Milestone #1: Complete genome sequencing of virus X. Milestone #2: Construction of virus clones with selected knockout mutations. Milestone #3: Viability testing of knockout mutants in cell culture. Milestone #4: In vivo challenge with viable mutants. Milestone #5: Comprehensive analysis of pathogenesis and immune response to each mutant. Milestone #6: Identification of corporate partners interested in vaccine for virus X. 3/1/2008 2/28/2009 3/1/2008 6/1/2008 6/1/2008 9/1/2008 2 9/1/2008 11/1/2008 2,3 11/1/2008 1/1/2009 2,3,4 1/1/2009 2/28/2009 2,3,4,5 8/1/08 2/28/09 2,3,4,(5) 2 3 4 5 6 7 Dependencies Task Name is the description of event to occur (e.g., clone gene into vector, sequence gene, analyze expression, purify protein, vaccinate animal, bleed day, etc.) Dependencies: Which tasks must precede the current task Submit applications to: Kimberly Schuenke, PhD WRCE Program Administrator The University of Texas Medical Branch G.170 Keiller Bldg. 301 University Blvd. Galveston, TX 77555-0609 phone: (409) 747-0766 kischuen@utmb.edu For further information, please contact: Douglas M. Watts, PhD WRCE Assoc. Director The University of Texas at El Paso 500 W. University Ave. Biosciences Bldg. 2.120 El Paso, TX 79968 phone: (915) 747-8715 fax: (915) 747-5808 dowatts@utmb.edu Kimberly Schuenke, PhD WRCE Program Administrator The University of Texas Medical Branch phone: (409) 747-0766 fax: (409) 747-0762 kischuen@utmb.edu 3