Foiled for the Last Time! Purpose - Mini
advertisement

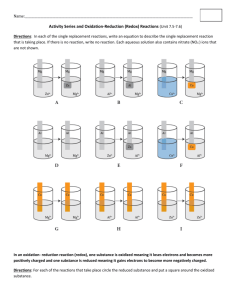
Mini-Lab 6-06: Foiled for the Last Time! Purpose: What if you expect something to happen, but it doesn’t? This is an exercise in thinking “outside the box”. Think about this: When there is a positive potential difference between two substances, the reaction will be spontaneous. Reduction Reaction Cu2+ + 2e- Cu(s) Al3+ + 3e- Al(s) Reduction Potential 0.34 V -1.66 V Safety 1) 2) 3) 4) Don’t eat or drink anything in the lab. Always wear eye protection. Wear protective clothing (lab coats, etc.). Don’t play around – treat the lab with respect. Questions Place one small scoop of copper chloride crystals onto a piece of Al foil. 1) What do you observe happening? 2) What is the electrochemical potential difference between copper and aluminum? Do you expect a reaction to occur? If so, write a balanced equation for it. 3) Grind the copper chloride crystals onto the aluminum foil to create more contact between them. What do you observe? 4) Add a few drops of water to the pile of crystals. What do you observe? WAIT! Do not write down an answer to the Final question until your Instructor tells you to. 5) Explain what happened in step 3, to the best of your ability. Why do you think there was no reaction initially? Instructor’s Page 6-06: Foiled for the Last Time! Concepts: potential difference, spontaneity Materials: Aluminum foil, copper chloride salt dihydrate (Alternatively, copper sulfate can be used, and a solution of about 1 M NaCl can be poured over the copper sulfate. Root Killer for pouring down your drains is 99% copper sulfate and can be purchased at any hardware store.) Hints: Under these conditions, this reaction is even more astonishing than it was when observed under solution conditions in Mini-Lab 1-03. They should realize that the following reaction has a potential difference of 2.00V, and thus should be spontaneous: 3CuCl2(aq) + 2Al(s) → 3Cu(s) + 2AlCl3(aq) What they probably do not realize is that there is a molecularly thin layer of aluminum oxide (Al2O3) on the surface of the foil. Since aluminum has such a high oxidation potential (+1.66 V), it spontaneously oxidizes on exposure to air. Aluminum oxide is a very hard and resistant material (also known as “sapphire”), second in hardness only to diamond. However, adding water allows the chloride ions to separate from the copper ions. Chloride associates with aluminum ions very strongly – strongly enough that it “rips” the aluminum ions away from the oxygen ions in the aluminum oxide layer, allowing pure aluminum to come in contact with the copper ions. The result is a very rapid and startling conversion of copper ions to copper metal, with the associated conversion of aluminum metal to aluminum ions. A take-away lesson here is this: when you expect something to occur, and it doesn’t, don’t abandon basic principles. Instead, do some additional investigation to find out what’s going on. For More Information: Any good general chemistry text will have information on aluminum, as well as electrochemistry. Both of these subjects would be informative to understanding this activity.