ele12355-sup-0002-Appendix2
advertisement
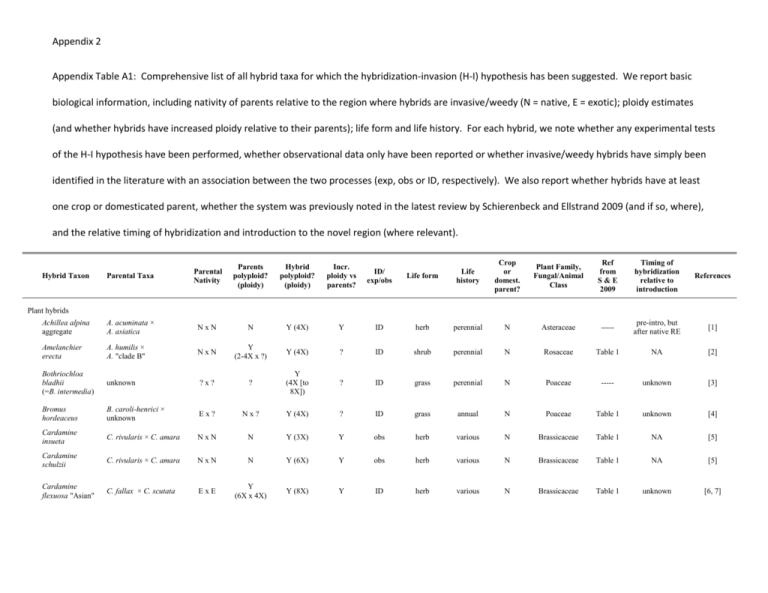
Appendix 2 Appendix Table A1: Comprehensive list of all hybrid taxa for which the hybridization-invasion (H-I) hypothesis has been suggested. We report basic biological information, including nativity of parents relative to the region where hybrids are invasive/weedy (N = native, E = exotic); ploidy estimates (and whether hybrids have increased ploidy relative to their parents); life form and life history. For each hybrid, we note whether any experimental tests of the H-I hypothesis have been performed, whether observational data only have been reported or whether invasive/weedy hybrids have simply been identified in the literature with an association between the two processes (exp, obs or ID, respectively). We also report whether hybrids have at least one crop or domesticated parent, whether the system was previously noted in the latest review by Schierenbeck and Ellstrand 2009 (and if so, where), and the relative timing of hybridization and introduction to the novel region (where relevant). Parental Taxa Parental Nativity Parents polyploid? (ploidy) Hybrid polyploid? (ploidy) Incr. ploidy vs parents? ID/ exp/obs Life form Life history Crop or domest. parent? Plant Family, Fungal/Animal Class Ref from S&E 2009 Timing of hybridization relative to introduction References Achillea alpina aggregate A. acuminata × A. asiatica NxN N Y (4X) Y ID herb perennial N Asteraceae ----- pre-intro, but after native RE [1] Amelanchier erecta A. humilis × A. "clade B" NxN Y (2-4X x ?) Y (4X) ? ID shrub perennial N Rosaceae Table 1 NA [2] Bothriochloa bladhii (=B. intermedia) unknown ?x? ? Y (4X [to 8X]) ? ID grass perennial N Poaceae ----- unknown [3] Bromus hordeaceus B. caroli-henrici × unknown Ex? Nx? Y (4X) ? ID grass annual N Poaceae Table 1 unknown [4] Cardamine insueta C. rivularis × C. amara NxN N Y (3X) Y obs herb various N Brassicaceae Table 1 NA [5] Cardamine schulzii C. rivularis × C. amara NxN N Y (6X) Y obs herb various N Brassicaceae Table 1 NA [5] Cardamine flexuosa "Asian" C. fallax × C. scutata ExE Y (6X x 4X) Y (8X) Y ID herb various N Brassicaceae Table 1 unknown [6, 7] Hybrid Taxon Plant hybrids Hybrid Taxon Parental Taxa Parental Nativity Parents polyploid? (ploidy) Hybrid polyploid? (ploidy) Incr. ploidy vs parents? ID/ exp/obs Life form Life history Crop or domest. parent? Plant Family, Fungal/Animal Class Ref from S&E 2009 Timing of hybridization relative to introduction References Carpobrotus hybrids C. edulis × C. chilensis ExE N N N exp shrub perennial N Aizoaceae Table 2 post-intro [8-10] Carpobrotus × cf. acinaciformis C. edulis × C. acinaciformis ExE N N N exp* shrub perennial N Aizoaceae ----- unknown [11] Carthamus creticus C. lanatus × C. leucocaulos NxN Y/N (4X x 2X) Y (6X) Y ID herb various N Asteraceae ----- pre-intro, but after native RE [12] Carthamus turkestanicus C. lanatus × C. glaucus NxN Y/N (4X x 2X) Y (6X) Y ID herb various N Asteraceae ----- pre-intro, but after native RE [12] Casuarina hybrids C. equisetifolia × C. glauca and/or C. cunninghamiana ExE N N N ID tree perennial N Casuarinaceae ----- post-intro [13] Centaurea hybrids C. diffusa × C. stoebe (=C. maculosa) ExE N/Y (2X x 24X) N N obs herb various N Asteraceae ----- pre-intro [14, 15] Centaurea x monchtonii C. nigra × C. jacea ExE Y (4X x 4X) Y (4X) N obs herb perennial N Asteraceae ----- pre-intro [16] Centaurea stoebe s. l. C. stoebe s. str. × unknown Ex? N/? (4X x 2X) Y (4X) Y exp? herb various N Asteraceae ----- pre-intro, but after native RE [17] Circaea x intermedia C. alpina × C. lutetiana NxN N N N ID herb perennial N Onagraceae Table 1 NA [18, 19] Glyceria x pedicellata G. fluitans × G. notata NxN Y/N? (8X x ?) N N ID grass perennial N Poaceae Table 1 unknown [19, 20] Helianthus annuus subsp. texanus H. annuus × H. debilis NxN N N N exp herb annual N Asteraceae Table 1 NA [21] Hieracium pilosella-like hybrids H. pilosella × H. praealtum ExE Y (4X x 4X) Y (4-5X) N ID herb perennial N Asteraceae ----- post-intro [22] Hieracium x stoloniflorum H. aurantiacum × H. pilosella ExE Y (4X x 4X?) Y (6X) Y ID herb perennial N Asteraceae ----- pre-intro [23] Lactuca hybrids L. serriola × L. sativa NxE N N N exp herb annual Y Asteraceae ----- post-intro [24] Hybrid Taxon Parental Taxa Parental Nativity Parents polyploid? (ploidy) Hybrid polyploid? (ploidy) Incr. ploidy vs parents? ID/ exp/obs Life form Life history Crop or domest. parent? Plant Family, Fungal/Animal Class Ref from S&E 2009 Timing of hybridization relative to introduction References Mentha x verticillata M. aquatica × M. arvensis NxN Y (8X x 6X) Y (7X) N ID herb perennial N Lamiaceae Table 1 NA [20] Myriophyllum heterophyllum hybrids M. heterophyllum × M. laxum ExE N N N obs herb perennial N Haloragaceae Table 2 pre-intro [25, 26] Myriophyllum spicatum hybrids M. spicatum × M. sibiricum E x E/N Y (6X x 6X) unknown unknown exp herb perennial N Haloragaceae ----- post-intro [27] Nasturtium x sterile (=Rorippa x sterilis) N. microphyllum × N. officinale (=R. microphyllum × R. nasturtium-aquaticum) NxN Y (8X x 4X) Y (6X) N obs herb perennial Y? Brassicaceae Table 1 NA [18, 28, 29] Nuphar x spenneriana (=N. x intermedia) N. lutea × N. pumila subsp. pumila NxN N N N ID herb perennial N Nymphaeaceae ----- NA [30] Oenothera glazioviana O. hookeri (=O. elata) × O. biennis ExE N N N ID herb biennial N Onagraceae Table 1 post-intro, but subsequent introductions [31, 32] Onopordum hybrids O. acanthium × O. illyricum ExE N/N? (2X x 2X) N N ID herb biennial N Asteraceae Table 2 pre-intro [33] Persicaria maculosa (=Polygonum persicaria) P. foliosa × P. lapathifolia ExE N Y (4X) Y ID herb annual N Polygonaceae ----- pre-intro, but after native RE [34] Persicaria punctata P. hydropiper × P. hirsuta/setacea ExE N Y (4X) Y ID herb various N Polygonaceae ----- pre-intro [34] Persicaria pensylvanica (=Polygonum pensylvanicum) P. glabra/hispida × unknown unknown (Y/N)/? (2X or 46X x ?) Y (8X) Y ID herb various N Polygonaceae ----- unknown [34] Parental Nativity Parents polyploid? (ploidy) Hybrid polyploid? (ploidy) Incr. ploidy vs parents? ID/ exp/obs Life form Life history Crop or domest. parent? Plant Family, Fungal/Animal Class Ref from S&E 2009 Timing of hybridization relative to introduction References R.raphanistrum × R. sativus ExE N N N exp herb annual Y Brassicaceae Table 2 post-intro [35, 36] Reynoutria x bohemia (=Fallopia x bohemica) R. japonica × R. sachalinensis (=F. japonica × F. sachalinensis) ExE Y (4/6/8X x 4/8X) Y (6X [4/8X]) N exp herb perennial N Polygonaceae Table 1 post-intro, but subsequent introductions [37, 38] Rhododendron hybrids R. ponticum × R. catawbiense ExE N N N ID shrub perennial N Ericaceae Table 2 post-intro [39] Rorippa x armoracioides R. sylvestris × R.austriaca NxE N/Y (4/6X x 2X) Y (3-5X) N obs herb perennial N Brassicaceae Table 1 post-intro, but subsequent introductions [40] Salsola Type C S. tragus × S. kali ssp. Austroafricanus ExE Y/N (4X x 2X) Y (6X [4X]) Y (N) ID herb annual N Chenopodiaceae Table 1 post-intro [41] Salsola lax S. tragus × S. kali ssp. Austroafricanus × S. paulsenii (?) ExE Y/N/Y (6X x 2X x 6X) Y (6X) N ID herb annual N Chenopodiaceae ----- unknown [41] Sarcocornia hybrids S. perennis × S. fruticosa NxN Y/N (2X x 8X) Y (6X [45X]) N exp shrub perennial N Chenopodiaceae Table 2 NA [42, 43] Senecio vulgaris var. hibernicus S. squalidus × S. vulgaris var. vulgaris ExN N/Y (2X x 4X) Y (4X) N exp herb various N Asteraceae Table 1 post-intro [44] Senecio squalidus S. aethnensis × S. chrysanthemumifolius ExE N N N exp herb various N Asteraceae Table 1 pre-intro [45] Solanum x edinense S. tuberosum ssp. andigena × S. demissum NxN Y (4X x 6X) Y (5X) N ID herb perennial Y Solanaceae Table 1 NA [46, 47] Solanum x rechei S. microdontum × S. kurtzianum NxN N N N ID herb perennial N Solanaceae ----- NA [47, 48] Solanum x sambucinum S. ehrenbergii × S. pinnatisectum NxN N N N ID herb perennial N Solanaceae ----- NA [47] Sorghum almum S. halepense × S. bicolor ExE N/Y (4X x 2X) Y (4X) N exp grass perennial Y Poaceae Table 1 post-intro [49] Hybrid Taxon Parental Taxa Raphanus hybrids Hybrid Taxon Parental Taxa Parental Nativity Parents polyploid? (ploidy) Hybrid polyploid? (ploidy) Incr. ploidy vs parents? ID/ exp/obs Life form Life history Crop or domest. parent? Plant Family, Fungal/Animal Class Ref from S&E 2009 Timing of hybridization relative to introduction References Sorghum halepense S. bicolor × S. propiniquum ExE N Y (4X) Y ID grass perennial Y Poaceae Table 1 post-intro, but subsequent introductions [50] Spartina hybrids S. alterniflora × S. foliosa ExN Y (6X x 6X) Y (6X) N exp grass perennial N Poaceae Table 2 post-intro [51, 52] Spartina anglica S. maritima × S. alterniflora NxE N Y (12X) Y ID grass perennial N Poaceae Table 1 post-intro, but subsequent introductions [53] Spartina densiflora S. arundinacea × S. alterniflora NxN Y (4X x 6X) Y (7X) Y ID grass perennial N Poaceae ----- NA [54] Sphagneticola hybrids S. trilobata × S. calendulacea ExN Y (4X x 4X) Y (4X) N exp herb perennial N Asteraceae ----- post-intro [55] Stachys x ambigua S. palustris × S. sylvatica NxN Y (12-13X x 8X) Y (10X) N ID herb perennial N Lamiaceae Table 1 NA [19] Symphytum x uplandicum S. officinale × S. asperum NxE Y (4X x 2X) Y (3X+) N ID herb perennial N Boraginaceae ----- pre-intro [56, 57] Tamarix hybrids T. ramosissima × T. chinensis ExE N N N obs shrub perennial N Tamaricaceae Table 2 post-intro [58, 59] Tragopogon mirus T. dubius × T. porrifolius ExE N Y (4X) Y ID herb biennial N Asteraceae Table 1 post-intro [60, 61] Tragopogon miscellus T. dubius × T. pratensis ExE N Y (4X) Y ID herb biennial N Asteraceae Table 1 post-intro [60, 61] Typha x glauca T. angustifolia × T. latifolia ExN N N N exp graminoid perennial N Typhaceae Table 1 post-intro [62, 63] Ulmus hybrids U. rubra × U. pumila NxE N N N ID tree perennial Y Ulmaceae ----- post-intro [64] Viola hybrids V. riviana × V. reichenbachiana NxN Y/N (4X x 2X) Y (4X) N ID herb perennial N Violaceae Table 2 NA [65] Viola x tatrae V. tricolor × V. lutea ssp. sudetica ExN Y (4X x 8X) Y (6X and up) N exp herb perennial N Violaceae ----- post-intro [66] Hybrid Taxon Parental Taxa Parental Nativity Parents polyploid? (ploidy) Hybrid polyploid? (ploidy) Incr. ploidy vs parents? ID/ exp/obs Life form Life history Crop or domest. parent? Plant Family, Fungal/Animal Class Ref from S&E 2009 Timing of hybridization relative to introduction References Wisteria hybrids W. floribunda × W. sinensis ExE N N N obs vine perennial Y Fabaceae ----- post-intro, but subsequent introductions [67] Melampsora x columbiana M. medusae × M. occidentalis N/E x N ? N exp fungus NA N Urediniomycetes ----- post-intro [68, 69] Ophiostoma novo-ulmi O. novo-ulmi × O. ulmi ExE ? N ID fungus NA N Sordariomycetes ----- post-intro, but subsequent introductions [70, 71] Phytophthora alni alni P. alni uniformis × P. alni multiformis N? x E? N Y ID stramenopile NA N Oomycetes Table 4 post-intro [72, 73] Phytophthora andina P. infestans × unknown E x ?? ? N ID stramenopile NA N Oomycetes ----- unknown [74] Phytophthora x pelgrandis P. cactorum × P. nicotianae NxE ? N ID stramenopile NA N Oomycetes ----- unknown [75] Phytophthora x serendipita P. cactorum × P. hedraiandra NxE ? N ID stramenopile NA N Oomycetes ----- unknown [76] Pythium hybrids P. arrhenomanes (or similar) × P. phragmitis ExN ? N exp stramenopile NA N Oomycetes ----- unknown [77] Verticillium longisporum V. dahliaea × unknown ?? X ?? N Y (2X) ID fungus NA N Ascomycota ----- unknown [78] Ambystoma hybrids A. tigrinum mavortium × A. californiense ExN ? N exp amphibian NA N Amphibia ----- post-intro [79, 80] Carcinus hybrids C. maenas × C. aestuarii ExE N N N ID crab NA N Malacostraca ----- unknown [81, 82] Cottus hybrids C. rhenanus × C. perifretum NxN ? N N? obs fish NA N Teleostei ----- post-intro [83] Cyprinella hybrids C. lutrensis × C. venusta stigmatura ExN ? N exp fish NA N Actinopterygii ----- post-intro [84] Salvelinus hybrids S. fontinalis × S. alpinus NxN ? N ID fish NA N Actinopterygii ----- post-intro [85] Fungal hybrids Y Animal hybrids References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Guo, Y.P., et al., Hybrid origin and differentiation of two tetraploid Achillea species in East Asia: molecular, morphological and ecogeographical evidence. Molecular Ecology, 2006. 15(1): p. 133-144. Campbell, C.S., et al., Persistent nuclear ribosomal DNA sequence polymorphism in the Amelanchier agamic complex (Rosaceae). Molecular Biology and Evolution, 1997. 14(1): p. 81-90. Harlan, J.R. and J.M.J. de Wet, The compilospecies concept. Evolution, 1963. 17(4): p. 497-501. Ainouche, M.L., et al., The allotetraploid invasive weed Bromus hordeaceus L. (Poaceae): Genetic diversity, origin and molecular evolution. Folia Geobotanica, 1999. 34(4): p. 405-419. Urbanska, K.M., et al., Hybridization and evolution in Cardamine (Brassicaceae) at Urnerboden, central Switzerland: Biosystematic and molecular evidence. Plant Systematics and Evolution, 1997. 204(3-4): p. 233-256. Lihova, J., et al., Worldwide phylogeny and biogeography of Cardamine flexuosa (Brassicaceae) and its relatives. American Journal of Botany, 2006. 93(8): p. 1206-1221. Bleeker, W., et al., DNA sequences identify invasive alien Cardamine at Lake Constance. Biological Conservation, 2008. 141(3): p. 692-698. Vila, M. and C.M. D'Antonio, Hybrid vigor for clonal growth in Carpobrotus (Aizoaceae) in coastal California. Ecological Applications, 1998. 8(4): p. 1196-1205. Vila, M. and C.M. D'Antonio, Fitness of invasive Carpobrotus (Aizoaceae) hybrids in coastal California. Ecoscience, 1998. 5(2): p. 191-199. Vila, M., E. Weber, and C.M. D'Antonio, Flowering and mating system in hybridizing Carpobrotus (Aizoaceae) in coastal California. Canadian Journal of Botany-Revue Canadienne De Botanique, 1998. 76(7): p. 1165-1169. Van Grunsven, R.H.A., et al., Release from soil pathogens plays an important role in the success of invasive Carpobrotus in the Mediterranean. South African Journal of Botany, 2009. 75(1): p. 172-175. Vilatersana, R., A.K. Brysting, and C. Brochmann, Molecular evidence for hybrid origins of the invasive polyploids Carthamus creticus and Cturkestanicus (Cardueae, Asteraceae). Molecular Phylogenetics and Evolution, 2007. 44(2): p. 610-621. Gaskin, J.F., et al., Molecular evidence of hybridization in Florida's sheoak (Casuarina spp.) invasion. Molecular Ecology, 2009. 18(15): p. 32163226. Blair, A.C. and R.A. Hufbauer, Hybridization and invasion: one of North America's most devastating invasive plants shows evidence for a history of interspecific hybridization. Evolutionary Applications, 2010. 3(1): p. 40-51. Blair, A.C. and R.A. Hufbauer, Geographic Patterns of Interspecific Hybridization between Spotted Knapweed (Centaurea stoebe) and Diffuse Knapweed (C. diffusa). Invasive Plant Science and Management, 2009. 2(1): p. 55-69. Roche, C.T. and B.F. Roche, Meadow knapweed invasion in the Pacific Northwest, United States of America and British Columbia, Canada Northwest Science, 1991. 65(1): p. 53-61. Mraz, P., et al., Allopolyploid origin of highly invasive Centaurea stoebe s.l. (Asteraceae). Molecular Phylogenetics and Evolution, 2012. 62(2): p. 612-623. Raven, P.H., Circaea in the British Isles. Watsonia, 1963. 5(5): p. 262-272. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. Stace, C.E., Hybridization and the Flora of the British Isles. 1975, London: Academic Press. Stace, C.E., New Flora of the British Isles. 1991, Cambridge: Cambridge University Press. Whitney, K.D., R.A. Randell, and L.H. Rieseberg, Adaptive introgression of herbivore resistance traits in the weedy sunflower Helianthus annuus. American Naturalist, 2006. 167(6): p. 794-807. Morgan-Richards, M., et al., Interspecific hybridization among Hieracium species in New Zealand: evidence from flow cytometry. Heredity, 2004. 93(1): p. 34-42. Fernandez, M. and C. Ezcurra, Hieracium x stoloniflorum (Asteraceae, Lactuceae), exotic weed new for Argentina. Darwiniana, 2009. 47(2): p. 339-343. Hooftman, D.A.P., et al., Demographic vital rates determine the performance advantage of crop-wild hybrids in lettuce. Journal of Applied Ecology, 2005. 42(6): p. 1086-1095. Moody, M.L. and D.H. Les, Evidence of hybridity in invasive watermilfoil (Myriophyllum) populations. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(23): p. 14867-14871. Thum, R.A. and J.T. Lennon, Is hybridization responsible for invasive growth of non-indigenous water-milfoils? Biological Invasions, 2006. 8(5): p. 1061-1066. LaRue, E.A., et al., Hybrid watermilfoil lineages are more invasive and less sensitive to a commonly used herbicide than their exotic parent (Eurasian watermilfoil). Evolutionary Applications, 2013. 6(3): p. 462-471. Manton, I., The cytological history of Watercress (Nasturtium officinale R. Br.). Molecular and General Genetics, 1935. 69(1): p. 132-157. Bleeker, W., M. Huthmann, and H. Hurka, Evolution of hybrid taxa in Nasturtium R.Br. (Brassicaceae). Folia Geobotanica, 1999. 34(4): p. 421-433. Heslop-Harrison, Y., Nuphar intermedia Ledeb., a presumed relict hybrid, in Britain. Watsonia, 1953. 3: p. 7-25. Dietrich, W., W.L. Wagner, and P.H. Raven, Systematics of Oenothera Section Oenothera Subsection Oenothera (Onagraceae). Systematic Botany Monographs, 1997. 50: p. 1-234. Raven, P.H., W. Dietrich, and W. Stubbe, An outline of the systematics of Oenothera subsect Euoenothera (Onagraceae) Systematic Botany, 1979. 4(3): p. 242-252. O'Hanlon, P.C., R. Peakall, and D.T. Briese, Amplified fragment length polymorphism (AFLP) reveals introgression in weedy Onopordum thistles: hybridization and invasion. Molecular Ecology, 1999. 8(8): p. 1239-1246. Kim, S.T., S.E. Sultan, and M.J. Donoghue, Allopolyploid speciation in Persicaria (Polygonaceae): Insights from a low-copy nuclear region. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(34): p. 12370-12375. Campbell, L.G., A.A. Snow, and C.E. Ridley, Weed evolution after crop gene introgression: greater survival and fecundity of hybrids in a new environment. Ecology Letters, 2006. 9(11): p. 1198-1209. Ridley, C.E. and N.C. Ellstrand, Evolution of enhanced reproduction in the hybrid-derived invasive, California wild radish (Raphanus sativus). Biological Invasions, 2009. 11(10): p. 2251-2264. Pysek, P., et al., Vegetative regeneration in invasive Reynoutria (Polygonaceae) taxa: The determinant of invasibility at the genotype level. American Journal of Botany, 2003. 90(10): p. 1487-1495. Parepa, M., et al., Hybridization increases invasive knotweed success. Evolutionary Applications, 2014. 7(3): p. 413-420. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. Milne, R.I. and R.J. Abbott, Origin and evolution of invasive naturalized material of Rhododendron ponticum L. in the British Isles. Molecular Ecology, 2000. 9(5): p. 541-556. Bleeker, W. and A. Matthies, Hybrid zones between invasive Rorippa austriaca and native R-sylvestris (Brassicaceae) in Germany: ploidy levels and patterns of fitness in the field. Heredity, 2005. 94(6): p. 664-670. Ayres, D., et al., Tumbleweed (Salsola, section Kali) species and speciation in California. Biological Invasions, 2009. 11(5): p. 1175-1187. Figueroa, M.E., et al., Facilitated invasion by hybridization of Sarcocornia species in a salt-marsh succession. Journal of Ecology, 2003. 91(4): p. 616-626. Redondo, S., et al., Influences of salinity and light on germination of three Sarcocornia taxa with contrasted habitats. Aquatic Botany, 2004. 78(3): p. 255-264. Hawkes, C.V., A.E. Douglas, and A.H. Fitter, Origin, local experience, and the impact of biotic interactions on native and introduced Senecio species. Biological Invasions, 2010. 12(1): p. 113-124. Brennan, A.C., et al., Molecular genetic and quantitative trait divergence associated with recent homoploid hybrid speciation: a study of Senecio squalidus (Asteraceae). Heredity, 2012. 108(2): p. 87-95. Ugent, D., Morphological variation in Solanum x edinense, a hybrid of the common potato. Evolution, 1967. 21(4): p. 696-712. Hawkes, J.G., The potato: evolution, biodiversity and genetic resources. 1990, London: Bellhaven Press. Clausen, A.M. and D.M. Spooner, Molecular support for the hybrid origin of the wild potato species Solanum x rechei. Crop Science, 1998. 38(3): p. 858-865. Arriola, P.E. and N.C. Ellstrand, Fitness of interspecific hybrids in the genus Sorghum: Persistence of crop genes in wild populations. Ecological Applications, 1997. 7(2): p. 512-518. Paterson, A.H., et al., The weediness of wild plants - Molecular analysis of genes influencing dispersal and persistence of Johnsongrass, Sorghum Halepense (L.) Pers Proceedings of the National Academy of Sciences of the United States of America, 1995. 92(13): p. 6127-6131. Ayres, D.R., et al., Hybridization between invasive Spartina densiflora (Poaceae) and native S-foliosa in San Francisco Bay, California, USA. American Journal of Botany, 2008. 95(6): p. 713-719. Ayres, D.R., et al., Spread of exotic cordgrasses and hybrids (Spartina sp.) in the tidal marshes of San Francisco Bay, California, USA. Biological Invasions, 2004. 6(2): p. 221-231. Ainouche, M.L., et al., Hybridization, polyploidy and invasion: lessons from Spartina (Poaceae). Biological Invasions, 2009. 11(5): p. 1159-1173. Fortune, P.M., et al., The enigmatic invasive Spartina densiflora: A history of hybridizations in a polyploidy context. Molecular Ecology, 2008. 17(19): p. 4304-4316. Wu, W., et al., Is a new invasive herb emerging? Molecular confirmation and preliminary evaluation of natural hybridization between the invasive Sphagneticola trilobata (Asteraceae) and its native congener S-calendulacea in South China. Biological Invasions, 2013. 15(1): p. 75-88. Elkington, T.T., Cytogenetic variation in the British flora - origins and significance. New Phytologist, 1984. 98(1): p. 101-118. Sykes, W.R., Checklist of dicotyledons naturalized in New Zealand. 10. Polemoniales and Boraginaceae New Zealand Journal of Botany, 1981. 19(3): p. 311-317. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. Gaskin, J.F., A.S. Birken, and D.J. Cooper, Levels of novel hybridization in the saltcedar invasion compared over seven decades. Biological Invasions, 2012. 14(3): p. 693-699. Gaskin, J.F. and B.A. Schaal, Hybrid Tamarix widespread in US invasion and undetected in native Asian range. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(17): p. 11256-11259. Clements, D.R., M.K. Upadhyaya, and S.J. Bos, The biology of Canadian weeds. 110. Tragopogon dubius Scop., Tragopogon pratensis L., and Tragopogon porrifolius L. Canadian Journal of Plant Science, 1999. 79(1): p. 153-163. Novak, S.J., D.E. Soltis, and P.S. Soltis, Ownbey Tragopogons - 40 years later. American Journal of Botany, 1991. 78(11): p. 1586-1600. Sullivan, L., et al., Growth of three cattail (Typha) taxa in response to elevated CO(2). Plant Ecology, 2010. 207(1): p. 121-129. Travis, S.E., et al., Hybridization dynamics of invasive cattail (Typhaceae) stands in the Western Great Lakes Region of North America: a molecular analysis. Journal of Ecology, 2010. 98(1): p. 7-16. Zalapa, J.E., J. Brunet, and R.P. Guries, The extent of hybridization and its impact on the genetic diversity and population structure of an invasive tree, Ulmus pumila (Ulmaceae). Evolutionary Applications, 2010. 3(2): p. 157-168. Neuffer, B., et al., Spread of violets in polluted pine forests: morphological and molecular evidence for the ecological importance of interspecific hybridization. Molecular Ecology, 1999. 8(3): p. 365-377. Krahulcova, A., F. Krahulec, and J. Kirschner, Introgressive hybridization between a native and an introduced species: Viola lutea subsp sudetica versus V-tricolor. Folia Geobotanica & Phytotaxonomica, 1996. 31(2): p. 219-&. Trusty, J.L., et al., Identity of naturalised exotic Wisteria (Fabaceae) in the south-eastern United States. Weed Research, 2007. 47(6): p. 479-487. Newcombe, G., B. Stirling, and H.D. Bradshaw, Abundant pathogenic variation in the new hybrid rust Melampsora xcolumbiana on hybrid poplar. Phytopathology, 2001. 91(10): p. 981-985. Newcombe, G., et al., Melampsora xcolumbiana, a natural hybrid of M-medusae and M-occidentalis. Mycological Research, 2000. 104: p. 261274. Bates, M.R., K.W. Buck, and C.M. Brasier, Moleclular relationships between Ophiostoma ulmi and the NAN and EAN races of O. novo-ulmi determined by restriction fragment length polymorphisms of nuclear DNA. Mycological Research, 1993. 97: p. 449-455. Paoletti, M., K.W. Buck, and C.M. Brasier, Selective acquisition of novel mating type and vegetative incompatibility genes via interspecies gene transfer in the globally invading eukaryote Ophiostoma novo-ulmi. Molecular Ecology, 2006. 15(1): p. 249-262. Aguayo, J., et al., Strong Genetic Differentiation Between North American and European Populations of Phytophthora alni subsp uniformis. Phytopathology, 2013. 103(2): p. 190-199. Ioos, R., et al., Genetic characterization of the natural hybrid species Phytophthora alni as inferred from nuclear and mitochondrial DNA analyses. Fungal Genetics and Biology, 2006. 43(7): p. 511-529. Goss, E.M., et al., The Plant Pathogen Phytophthora andina Emerged via Hybridization of an Unknown Phytophthora Species and the Irish Potato Famine Pathogen, P. infestans. Plos One, 2011. 6(9). Nirenberg, H.I., W.F. Gerlach, and T. Graefenhan, Phytophthora Xpelgrandis, a new natural hybrid pathogenic to Pelargonium grandiflorum hort. Mycologia, 2009. 101(2): p. 220-231. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. in 't Veld, W.A.M., A.W.A.M. de Cock, and R.C. Summerbell, Natural hybrids of resident and introduced Phytophthora species proliferating on multiple new hosts. European Journal of Plant Pathology, 2007. 117(1): p. 25-33. Nechwatal, J. and K. Mendgen, Evidence for the occurrence of natural hybridization in reed-associated Pythium species. Plant Pathology, 2009. 58(2): p. 261-270. Inderbitzin, P., et al., The Ascomycete Verticillium longisporum Is a Hybrid and a Plant Pathogen with an Expanded Host Range. Plos One, 2011. 6(3). Fitzpatrick, B.M. and H.B. Shaffer, Hybrid vigor between native and introduced salamanders raises new challenges for conservation. Proceedings of the National Academy of Sciences of the United States of America, 2007. 104(40): p. 15793-15798. Ryan, M.E., J.R. Johnson, and B.M. Fitzpatrick, Invasive hybrid tiger salamander genotypes impact native amphibians. Proceedings of the National Academy of Sciences of the United States of America, 2009. 106(27): p. 11166-11171. Darling, J.A., Interspecific Hybridization and Mitochondrial Introgression in Invasive Carcinus Shore Crabs. Plos One, 2011. 6(3). Darling, J.A., et al., Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus. Molecular Ecology, 2008. 17(23): p. 4992-5007. Nolte, A.W., et al., An invasive lineage of sculpins, Cottus sp (Pisces, Teleostei) in the Rhine with new habitat adaptations has originated from hybridization between old phylogeographic groups. Proceedings of the Royal Society B-Biological Sciences, 2005. 272(1579): p. 2379-2387. Walters, D.M., et al., Red shiner invasion and hybridization with blacktail shiner in the upper Coosa River, USA. Biological Invasions, 2008. 10(8): p. 1229-1242. Doiron, S., L. Bernatchez, and P.U. Blier, A comparative mitogenomic analysis of the potential adaptive value of arctic charr mtDNA introgression in brook charr populations (Salvelinus fontinalis Mitchill). Molecular Biology and Evolution, 2002. 19(11): p. 1902-1909.







