Ch 7.5 Student Notes CD
advertisement

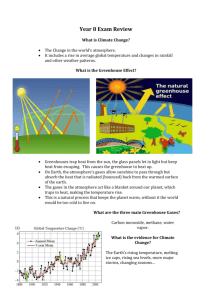
Environmental Science Name: ______________________________ Atmosphere and Climate Goal: The student will explain why the Earth’s atmosphere is like the glass in a greenhouse and explain why carbon dioxide levels in the atmosphere are rising. Vocabulary: 1. greenhouse gases Notes: Chapter 7.5: Greenhouse Earth and Global Warming • • When you get into a car in the summer, you may notice that it is hotter in the car than outside the car. This occurs when light energy from the sun streams in through the glass (absorbed by the carpet and upholstery and is changed into heat energy. Heat energy cannot flow back out through the glass, it gets trapped by the glass and continues to heat up This is the same principle which is applied when building a greenhouse for growing plants The Greenhouse Effect • Earth is similar to a greenhouse; outer space is icy cold, Earth’s atmosphere acts like the glass of a greenhouse trapping the heat from the sunlight • Heat radiates up from the Earth, some escapes into space but most is trapped by the gases in the troposphere and warms the air; this process is called the greenhouse effect. • Not all gases trap this heat, but the ones that do are called greenhouse gases (water vapor, carbon dioxide, chlorofluorocarbons (CFC’s), methane and nitrous oxide) • Water vapor and carbon dioxide are the most important Measuring Carbon Dioxide in Our Atmosphere • • • • 1958, Scientist, Charles Keeling installed instruments in Hawaii (Mauna Loa volcano) to measure the amount of CO2 in the air; winds blow pretty steady and have come thousands of miles across the Pacific Ocean By 1994, those levels had more drastic differences, summer levels did not fall as low as in 1958 and winter levels were higher (358 ppm) During photosynthesis, plants take in carbon dioxide, release oxygen; carbon is not returned to the air until the plant dies or the leaves fall Most of the CO2 released into the air dissolves in the ocean or is used by plants for photosynthesis; causing CO2 levels in the air to vary with the seasons; summer, CO2 levels decrease (plants use it), in winter, CO2 levels naturally rise Rising Carbon Dioxide Levels • In less than 50 years, CO2 levels in the atmosphere have risen by over 20% • Carbon is released into the air when burning these fossil fuels and when burning plants; millions of tons of carbon dioxide is released into the air from power plants • They have been able to determine CO2 levels in the atmosphere for thousands of years by analyzing ice cores drilled from ice sheets; much higher levels today than they have been for probably the last 20 million years. Greenhouse Gases and the Earth’s Temperature • Greenhouse gases are trapped near the Earth’s surface; scientists feel this will result in a warmer Earth. • Data collect over the last 400,000 years supports that view • Today, we are releasing more carbon dioxide into the atmosphere than any other greenhouse gas; power plants burn coal or oil, cars burn gasoline, millions of trees are being burned in tropical rainforests…all this is contributing to the increase of CO2 in our atmosphere • We are also releasing significant amounts of other greenhouse gases like CFC’s, methane and nitrous oxide • Scientist feel that with the increased gases we will be raising the Earth’s temperature by at least 2° by 2050 (global warming) Lesson Reflection: Using your books, on page 181, complete the handout How the Greenhouse Effect Works. Assessment: 1. Describe the process by which Chlorofluorocarbons destroy ozone molecules in the stratosphere. 2. Describe the process by which the ozone hole forms over Antarctica in spring. Lesson Extension (Technology/Application/Connection to Real World): Writing Assignment: Do you think the average seasonal temperatures have changed during the past few years? How do you think recent temperatures compare with temperatures from thousands or millions of years ago? How might a climate change affect Earth’s environment? YouTube Video: 6 Degrees Could Change the Earth (30 min)