EOR from Sandstones and Carbonates by “Smart Water”
advertisement

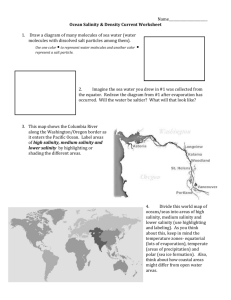
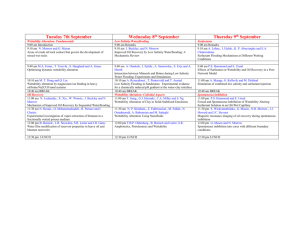
EOR from Sandstones and Carbonates by “Smart Water” The number of maturing oilfields in the world is steadily increasing, and because of that EOR (Enhanced Oil Recovery) is becoming more and more important. Constantly, different methods to be applied in different fields are being developed and explored, smart water injection being one of them. “Smart water” is a type of water having a certain beneficial composition of ions, and which is able to improve the oil recovery from a field upon water injection. What smart water does, is to disturb the chemical equilibrium, which is present between the rock, the oil and the brine inside the reservoir. This disturbance leads to wettability alteration, improvement of the wetting conditions of the reservoir, which affects the flow of oil and water inside the reservoir. The goal of the smart water injection is to improve the oil recovery beyond that of ordinary water injection. Sandstones Injection of a water of low salinity into sandstone reservoirs has been a topic for some decades, but it is just in recent years that this technology has been really focused on and implemented in field pilots and in some full scale processes. However, understanding why low salinity water injection in some cases functions well, and in other cases not at all, is not straightforward, and the mechanism behind the process is being investigated all over the world. At the University of Stavanger we have, on the basis of approximately four years of experimental work together with our industry partners, put forward a chemical mechanism for the low salinity process1-2. It is a two-step mechanism for wettability alteration of the clay minerals present in sandstones, and roughly explained it was suggested to involve: 1. A substitution of H+-ions from the water phase for the negative sites of the clay surface left after the initially attached Ca2+-ions desorb from the surface when the low salinity water (LS) water displaces the high salinity (HS) water. An alkaline environment close the clay surface is created because of an excess of OH- ions. 2. The acidic form of the adsorbed organic molecules onto the clay is, by an ordinary but fast acid-base reaction, transformed into the basic form, which has a lower affinity toward the clay surface, and is to some extent released from the surface. In order to observe LS EOR-effects, clay minerals must be preferential oil-wet, and adsorption of active polar oil components onto clay minerals is at a maximum close to their pKa values, at pH~5. At a given pH, the adsorption of organic components increases as the salinity decreases, due to the competition between the different active species towards the negative sites of the clay. Thus, a negative salinity gradient will increase adsorption of organic material onto the clay. If, however, the pH is increased simultaneously from 5-6 to about 8-9, desorption of organic material takes place, and the clay becomes more water-wet (Figure 1). 1 Figure 1 Adsorption of Quinoline vs. pH at ambient temperature for LS (1000 ppm) and HS (25000 ppm) fluids. These findings have also been confirmed by Fogden and Lebedeva3 in their study using crude oil and kaolinite clay. If, however, the initial pH of the formation brine is alkaline, pH>7, the clay will remain rather water-wet, and the potential for LS EOR-effects is low, as was observed for the Snorre pilot due to the presence of large amount of Plagioclase4-5. In addition to doing work for further documentation and understanding of the mechanism behind the extra oil recovery with low salinity water, we are in the starting phase of working out a method for screening the potential of a rock to respond to the low salinity EOR-process. Carbonates In carbonates, seawater (SW) is able to improve the water wetness at high temperatures and it can act as an EOR-fluid due to the presence of active potential determining ions, Ca2+, Mg2+, and SO42- in seawater. It has also been documented, that SW depleted in NaCl and/or spiked with some SO42- can act as an even better wettability modifier6-7(Figure 2). The chemical mechanism for the wettability alteration process is very well documented8, and it was seen that SO42- acts as a catalyst for the desorption of carboxylic material from the calcite/carbonate surface. Recently, we verified that low salinity EOR-effects can be observed in carbonates as well, provided that the formation contained anhydrite, CaSO4(s)9. Under the right conditions, a significant amount of extra oil can be produced in a tertiary LS flood. The chemical mechanism for the wettability alteration is in principles the same as for SW, but in this case, the catalytic species SO42- is obtained in situ by dissolution of anhydrite. 2 Figure 2. Spontaneous imbibition into oil saturated chalk cores at 90 ºC using VB (formation water), SW, and modified seawater: SW0NaCl, and SW0NaCl–4SO42–, Swi=10%, Oil–B, AN=0.5 mgKOH/g. Thus, in order to understand the chemical mechanism for the observed low salinity effect in carbonates, the impact of temperature and brine composition/salinity on the following equilibrium of precipitated, dissolved and adsorbed Ca2+ and SO42- must be understood: CaSO4(s) ↔ Ca2+(aq) + SO42-(aq) ↔ Ca2+(ad) + SO42-(ad) …………………… (1) What do we want to investigate next? Based on the knowledge of the principal mechanisms for the wettability modification in sandstones and carbonates, there are still questions to be asked and experimental work to be done. Below are some of the questions that need to be investigated in the near future. Sandstones o Is it possible to develop a simple method to test the potential of a reservoir core sample to respond to LS EOR-effects regarding oil recovery? How fast does it respond? o What is the link between temperature and formation brine salinity/composition regarding LS EOR-effect? o Will the presence of anhydrite in high temperature reservoirs suppress LS effects? o What are the optimal conditions for observing tertiary low salinity effects after flooding with SW? Carbonates o Can SW or modified SW act as wettability modifiers also in Dolomite? o Is it possible to observe LS effects in Dolomite containing anhydrite? o Speed of response to the LS fluid. Is it possible to observe LS effects by injection of a LS slug (a fraction of the pore volume) followed by HS water in 3 carbonates? The rate of the wettability alteration process decreases as the concentration of sulfate decreases, what is the impact? References (1) Austad, T.; RezaeiDoust, A.; Puntervold, T. In Chemical mechanism of low salinity water flooding in sandstone reservoirs, Paper SPE 129767 prepared for presentation at the 2010 SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA, 24-28 April, 2010. (2) RezaeiDoust, A.; Puntervold, T.; Austad, T., Energy & Fuels 2011, 25, 2151-2162. (3) Fogden, A.; Lebedeva, E. V. In Changes in wettability state due to waterflooding, The International Symposium of the Society of Core Analysts, Austin, TX, USA, 18-21 September, 2011. (4) Reinholdtsen, A. J.; RezaeiDoust, A.; Strand, S.; Austad, T. In Why such a small low salinity EOR - potential from the Snorre formation?, 16th European Symposium on Improved Oil Recovery, Cambridge, UK, 12-14 April, 2011. (5) Skrettingland, K.; Holt, T.; Tweheyo, M. T.; Skjevrak, I., SPE Reservoir Eval. & Eng. 2011, 14 (2), 182-192. (6) Fathi, S. J.; Austad, T.; Strand, S., Energy & Fuels 2010, 24, 2514-2519. (7) Fathi, S. J.; Austad, T.; Strand, S., Energy & Fuels 2011, 25, 5173-5179. (8) Zhang, P.; Tweheyo, M. T.; Austad, T., Colloids and Surfaces A: Physicochem. Eng. Aspects 2007, 301, 199-208. (9) Austad, T.; Shariatpanahi, S. F.; Strand, S.; Black, C. J. J.; Webb, K. J., Energy & Fuels 2012, 26 (1), 569-575. 4