Most colorectal cancers, regardless of etiology, arise from
advertisement

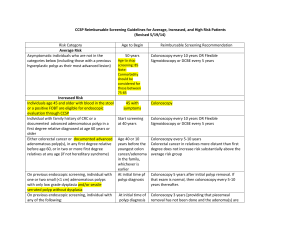
Colon Cancer SGD 1. EPIDEMIOLOGY - 2nd only to lung cancer as a cause of cancer death in the United States: 153,760 new cases occurred in 2007, and 52,180 deaths were due to colorectal cancer. - The incidence rate has remained relatively unchanged during the past 30 years, - Mortality rate has decreased, particularly in females. - Generally occurs in persons >50 years. 2. PATHOPHYSIOLOGY Polyps and Molecular Pathogenesis Most colorectal cancers, regardless of etiology, arise from adenomatous polyps. Polyp - a grossly visible protrusion from the mucosal surface nonneoplastic hamartoma (juvenile polyp) hyperplastic mucosal proliferation (hyperplastic polyp) adenomatous polyp – premalignant *only a minority of such lesions becomes cancer molecular changes: mutational activation of an oncogene followed by and coupled with the loss of genes that normally suppress tumorigenesis. 1. 2. 3. 4. 5. point mutations in the K-ras protooncogene hypomethylation of DNA, leading to gene activation loss of DNA (allelic loss) at the site of a tumor-suppressor gene [the adenomatous polyposis coli (APC) gene] on the long arm of chromosome 5 (5q21) allelic loss at the site of a tumor-suppressor gene located on chromosome 18q [the deleted in colorectal cancer (DCC) gene] allelic loss at chromosome 17p, associated with mutations in the p53 tumor-suppressor gene (see Fig. 79-2). Thus, the altered proliferative pattern of the colonic mucosa, which results in progression to a polyp and then to carcinoma, may involve the It remains uncertain whether the genetic aberrations always occur in a defined order. Based on this model, however, cancer is believed to develop only in those polyps in which most (if not all) of these mutational events take place. Clinically, the probability of an adenomatous polyp becoming a cancer depends on: 1. 2. 3. gross appearance of the lesion histologic features Size Adenomatous polyps: 1. pedunculated (stalked) 2. sessile (flat-based). a. Cancers develop more frequently in sessile polyp Histologically, adenomatous polyps may be 1. 2. 3. Tubular villous (i.e., papillary) a. most are sessile = become malignant more than three times as often as tubular adenomas tubulovillous Etiology and Risk Factors Risk Factors for the Development of Colorectal Cancer Diet: Animal fat Hereditary syndromes (autosomal dominant inheritance) Polyposis coli Nonpolyposis syndrome (Lynch syndrome) Inflammatory bowel disease Streptococcus bovis bacteremia Ureterosigmoidostomy ? Tobacco use Diet more often in upper socioeconomic populations Colorectal cancer has increased in Japan since that nation has adopted a more "western" diet Animal Fats animal fats found in red meats and processed meat leads to an increased proportion of anaerobes in the gut microflora resulting in the conversion of normal bile acids into carcinogens Insulin Resistance "western" diets with physical inactivity has been associated with a higher prevalence of obesity Obese persons insulin resistance with increased circulating levels of insulin > higher circulating concentrations of insulin-like growth factor type I (IGF-I) > stimulate proliferation of the intestinal mucosa Hereditary Factors and Syndromes Up to 25% of patients with colorectal cancer have a family history of the disease Hereditable (Autosomal Dominant) Gastrointestinal Polyposis Syndromes Syndrome Distribution of Polyps Histologic Type Malignant Potential Associated Lesions Familial adenomatous polyposis Large intestine Adenoma Common None Gardner's syndrome Large and small intestines Adenoma Common Osteomas, fibromas, lipomas, epidermoid cysts, ampullary cancers, congenital hypertrophy of retinal pigment epithelium Turcot's syndrome Large intestine Adenoma Common Brain tumors Nonpolyposis syndrome (Lynch syndrome) Large intestine (often proximal) Adenoma Common Endometrial and ovarian tumors Peutz-Jeghers syndrome Small and large intestines, stomach Hamartoma Rare Mucocutaneous pigmentation; tumors of the ovary, breast, pancreas, endometrium Juvenile polyposis Large and small intestines, stomach Hamartoma, rarely Rare progressing to adenoma 3. Various congenital abnormalities RISK FACTORS Diet - The etiology for most cases of large-bowel cancer appears to be related to environmental factors. - Occurs more often in upper socioeconomic populations who live in urban areas. - Mortality from colorectal cancer is directly correlated with per capita consumption of calories, meat protein, and dietary fat and oil as well as elevations in the serum cholesterol concentration and mortality from coronary artery disease. - Mormons and Seventh Day Adventists, whose lifestyle and dietary habits differ somewhat from those of their neighbors, have significantly lower-than-expected incidence and mortality rates for colorectal cancer. - Colorectal cancer has increased in Japan since that nation has adopted a more “western” diet. ANIMAL FATS - increased proportion of anaerobes in the gut microflora, resulting in the conversion of normal bile acids into carcinogens. INSULIN RESISTANCE - Obese persons develop insulin resistance with increased circulating levels of insulin, leading to higher circulating concentrations of insulin-like growth factor type I (IGF-I). This growth factor appears to stimulate proliferation of the intestinal mucosa. FIBER - Fibers failed to show any value for in preventing the recurrence of colorectal adenomas or the development of colorectal cancer. HEREDITARY FACTORS AND SYNDROMES - Up to 25% of patients with colorectal cancer have a family history of the disease, Divided into polyposis coli, and non-polyposis coli. Polyposis Coli - Appearance of thousands of adenomatous polyps throughout the large bowel. - autosomal dominant trait; - deletion in the long arm of chromosome 5 [including the APC (adenomatous polyposis coli) gene] – result in loss of inhibition of neoplastic growth. - Gardner’s syndrome: The presence of soft tissue and bony tumors, congenital hypertrophy of the retinal pigment epithelium, mesenteric desmoid tumors, and ampullary cancers in addition to the colonic polyps characterizes a subset of polyposis coli known as Gardner’s syndrome. The appearance of malignant tumors of the central nervous system accompanying polyposis coli - evident in affected individuals by age 25. - If the polyposis is not treated surgically, colorectal cancer will develop in almost all patients before age 40 - patients should undergo a total colectomy. - Medical therapy with nonsteroidal anti-inflammatory drugs ( - Proctosigmoidoscopy is a sufficient screening procedure Hereditary Nonpolyposis Colon Cancer - also known as Lynch syndrome, is another autosomal dominant trait. - presence of three or more relatives with histologically documented colorectal cancer, one of whom is a first-degree relative of the other two; one or more cases of colorectal cancer diagnosed before age 50 in the family; and colorectal cancer involving at least two generations. - appearance of an adenocarcinoma is <50 years, - better prognosis than sporadic tumors - undergo biennial colonoscopy beginning at age 25 years, - mutations of several genes, particularly hMSH2 on chromosome 2 and hMLH1 on chromosome 3. –leads to errors in DNA replication and results to abnormal cell growth and tumor development INFLAMMATORY BOWEL DISEASE - Large-bowel cancer is increased in incidence in patients with long-standing inflammatory bowel disease - Symptoms such as bloody diarrhea, abdominal cramping, and obstruction, which may signal the appearance of a tumor, are similar to the complaints caused by a flare-up of the underlying disease. - surgical removal of the colon can significantly reduce the risk for cancer OTHER HIGH-RISK CONDITIONS Streptococcus bovis Bacteremia - individuals who develop endocarditis or septicemia from this fecal bacterium have a high incidence of occult colorectal tumors Tobacco Use - Cigarette smoking is linked to the development of colorectal adenomas, particularly after >35 years 4. GUIDELINES FOR SCREENING COLON CANCER American Cancer Society Guidelines for the Early Detection of Colon Cancer Beginning at age 50, both men and women at average risk for developing colorectal cancer should use one of the screening tests below. The tests that are designed to find both early cancer and polyps are preferred if these tests are available to you and you are willing to have one of these more invasive tests. Talk to your doctor about which test is best for you. Tests that find polyps and cancer flexible sigmoidoscopy every 5 years* colonoscopy every 10 years double contrast barium enema every 5 years* CT colonography (virtual colonoscopy) every 5 years* Tests that mainly find cancer fecal occult blood test (FOBT) every year*,** fecal immunochemical test (FIT) every year*,** stool DNA test (sDNA), interval uncertain* *Colonoscopy should be done if test results are positive. **For FOBT or FIT used as a screening test, the take-home multiple sample method should be used. A FOBT or FIT done during a digital rectal exam in the doctor's office is not adequate for screening. People should talk to their doctor about starting colorectal cancer screening earlier and/or being screened more often if they have any of the following colorectal cancer risk factors: a personal history of colorectal cancer or adenomatous polyps a personal history of chronic inflammatory bowel disease (Crohns disease or ulcerative colitis) a strong family history of colorectal cancer or polyps (cancer or polyps in a first-degree relative [parent, sibling, or child] younger than 60 or in 2 or more first-degree relatives of any age) a known family history of hereditary colorectal cancer syndromes such as familial adenomatous polyposis (FAP) or hereditary non-polyposis colon cancer (HNPCC) Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C; Gastrointestinal Consortium Panel. Copyright © 2003 The American Gastroenterological Association. Published by Elsevier Science (USA) Difference from the 1997 guideline: 1. Against rehydrating fecal occult blood tests 2. The screening interval for double contrast barium enema has been shortened to 5 years 3. Colonoscopy is the preferred test for the diagnostic investigation of patients with findings on screening and for screening patients with a family history of hereditary nonpolyposis colorectal cancer 4. Recommendations for people with a family history of colorectal cancer make greater use of risk stratification 5. Guidelines for genetic testing are included 6. Guidelines for surveillance are also included. 7. Follow-up of postpolypectomy patients relies now on colonoscopy, and the first follow-up examination has been lengthened from 3 to 5 years for low-risk patients. If this were adopted nationally, surveillance resources could be shifted to screening and diagnosis. Promising new screening tests (virtual colonoscopy and tests for altered DNA in stool) are in development but are not yet ready for use outside of research studies. Despite a consensus among expert groups on the effectiveness of screening for colorectal cancer, screening rates remain low. Improvement depends on changes in patients' attitudes, physicians' behaviors, insurance coverage, and the surveillance and the surveillance and reminder systems necessary to support screening programs. 5. DIAGNOSIS •Digital rectal exam (DRE): The doctor inserts a lubricated, gloved finger into the rectum to feel for abnormal areas. It only detects tumors large enough to be felt in the distal part of the rectum but is useful as an initial screening test. •Fecal occult blood test (FOBT): a test for blood in the stool. Two types of tests can be used for detecting occult blood in stools i.e. guaiac based (chemical test) and immunochemical. The sensitivity of immunochemical testing is superior to that of chemical testing without an unacceptable reduction in specifity. •Endoscopy: –Sigmoidoscopy: A lighted probe (sigmoidoscope) is inserted into the rectum and lower colon to check for polyps and other abnormalities. –Colonoscopy: A lighted probe called a colonoscope is inserted into the rectum and the entire colon to look for polyps and other abnormalities that may be caused by cancer. A colonoscopy has the advantage that if polyps are found during the procedure they can be immediately removed. Tissue can also be taken for biopsy. Other methods of diagnosis •Double contrast barium enema (DCBE): First, an overnight preparation is taken to cleanse the colon. An enema containing barium sulfate is administered, then air is insufflated into the colon, distending it. The result is a thin layer of barium over the inner lining of the colon which is visible on X-ray films. A cancer or a precancerous polyp can be detected this way. This technique can miss the (less common) flat polyp. •Virtual colonoscopy replaces X-ray films in the double contrast barium enema (above) with a special computed tomography scan and requires special workstation software in order for the radiologist to interpret. This technique is approaching colonoscopy in sensitivity for polyps. However, any polyps found must still be removed by standard colonoscopy. •Standard computed axial tomography is an x-ray method that can be used to determine the degree of spread of cancer, but is not sensitive enough to use for screening. Some cancers are found in CAT scans performed for other reasons. •Blood tests: Measurement of the patient's blood for elevated levels of certain proteins can give an indication of tumor load. In particular, high levels of carcinoembryonic antigen (CEA) in the blood can indicate metastasis of adenocarcinoma. These tests are frequently false positive or false negative, and are not recommended for screening, it can be useful to assess disease recurrence. •Genetic counseling and genetic testing for families who may have a hereditary form of colon cancer, such as hereditary nonpolyposis colorectal cancer (HNPCC) or familial adenomatous polyposis (FAP). •Positron emission tomography (PET) is a 3-dimensional scanning technology where a radioactive sugar is injected into the patient, the sugar collects in tissues with high metabolic activity, and an image is formed by measuring the emission of radiation from the sugar. Because cancer cells often have very high metabolic rate, this can be used to differentiate benign and malignant tumors. PET is not used for screening and does not (yet) have a place in routine workup of colorectal cancer cases. •Whole-Body PET imaging is the most accurate diagnostic test for detection of recurrent colorectal cancer, and is a cost-effective way to differentiate resectable from non-resectable disease. A PET scan is indicated whenever a major management decision depends upon accurate evaluation of tumour presence and extent. •Stool DNA testing is an emerging technology in screening for colorectal cancer. Pre-malignant adenomas and cancers shed DNA markers from their cells which are not degraded during the digestive process and remain stable in the stool. Capture, followed by PCR amplifies the DNA to detectable levels for assay. Clinical studies have shown a cancer detection sensitivity of 71%–91%. •Carcinoembryonic antigen (CEA) is a protein found on virtually all colorectal tumors. CEA may be used to monitor and assess response to treatment in patients with metastatic disease. CEA can also be used to monitor recurrence in patients post-operatively. Staging •Colon cancer staging is an estimate of the amount of penetration of a particular cancer. It is performed for diagnostic and research purposes, and to determine the best method of treatment. The systems for staging colorectal cancers depend on the extent of local invasion, the degree of lymph node involvement and whether there is distant metastasis. •Definitive staging can only be done after surgery has been performed and pathology reports reviewed. An exception to this principle would be after a colonoscopic polypectomy of a malignant pedunculated polyp with minimal invasion. Preoperative staging of rectal cancers may be done with endoscopic ultrasound. Adjunct staging of metastasis include Abdominal Ultrasound, CT, PET Scanning, and other imaging studies. •The most common staging system is the TNM (for tumors/nodes/metastases) system, from the American Joint Committee on Cancer (AJCC). The TNM system assigns a number based on three categories. "T" denotes the degree of invasion of the intestinal wall, "N" the degree of lymphatic node involvement, and "M" the degree of metastasis. The broader stage of a cancer is usually quoted as a number I, II, III, IV derived from the TNM value grouped by prognosis; a higher number indicates a more advanced cancer and likely a worse outcome. AJCC stage TNM stage TNM stage criteria for colorectal cancer[39] Stage 0 Tis N0 M0 Tis: Tumor confined to mucosa; cancer-in-situ Stage I T1 N0 M0 T1: Tumor invades submucosa Stage I T2 N0 M0 T2: Tumor invades muscularis propria Stage II-A T3 N0 M0 T3: Tumor invades subserosa or beyond (without other organs involved) Stage II-B T4 N0 M0 T4: Tumor invades adjacent organs or perforates the visceral peritoneum Stage III-A T1-2 N1 M0 N1: Metastasis to 1 to 3 regional lymph nodes. T1 or T2. Stage III-B T3-4 N1 M0 N1: Metastasis to 1 to 3 regional lymph nodes. T3 or T4. Stage III-C any T, N2 M0 N2: Metastasis to 4 or more regional lymph nodes. Any T. Stage IV any T, any N, M1 M1: Distant metastases present. Any T, any N. Duke’s Classification •is an older and less complicated staging system, that predates the TMN system, and was first proposed by Dr. Cuthbert Dukes in 1932; it identifies the stages as •A - Tumour confined to the intestinal wall •B - Tumour invading through the intestinal wall •C - With lymph node(s) involvement (this is further subdivided into C1 lymph node involvement where the apical node is not involved and C2 where the apical lymph node is involved) •D - With distant metastasis 5. TREATMENT Total resection of tumor- optimal treatment for a malignant lesion in the large bowel Work up: evaluation for presence of metastatic disease, including a thourough PE, chest radiograph, biochemical assessment of liver function, and measurement of plasma CEA levels before surgery Colonoscopy of entire large bowel to identify synchronous neoplasms and polyps Detection of bleeding- usually an indication for a less radical surgery Laparotomy: inspection of entire peritoneal cavity, thorough inspection of the liver, pelvis and hemidiaphragm, careful palpation of the full length of the large bowel After complete resection: observed for 5 years, semiannual PE, yearly blood chemistry If with no complete colonoscopy: carry out within first several postoperative months Measurement of plasma CEA levels: 3 month intervals (sensitive for recurrence) Endoscopic and radiographic surveillance: triannually Abdominal CT scan: unreliable in assessing early recurrences Radiation therapy (pelvic): for patients with rectal cancer, reduces risk by 20-25% of recurrence, but it does not prolong survival (pre and post) o Preop- indicated for px with large, potentially unresectable recal cancers (shrink before surgery) o NOT effective in the primary treatment of cancer Reasons for success of radiation therapy in preventing pelvic recurrences Contained anatomical space within the pelvis limits the extent of the resection the rich lymphatic network of the pelvic side wall immediately adjacent to the rectum facilitates the early spread of malignant cells into surgically inaccessible tissue Total mesorectal excision: reduce local disease recurrence to 10% Systemic therapy 5-FU- remains the backbone of treatment (IV and oral) 5-FU + folinic acid (leucovorin)- improves efficacy of 5-FU in patients with advanced colorectal cancer by enhancing the binding of 5-FU to its target enzyme, thymidilate synthase Irinotecan (CPT-11)- topoisomerase 1 inhibitor, prolongs survival when compared to supportive care whose disease has progressed on 5-FU o Side effect: diarrhea 5-FU + Irinotecan + folinic acid- improves response rate and survival of px with metastatic disease 5-FU + Irinotecan + oxaliplatin- for metastatic disease (FOLFOX) Monoclonal antibodies- for advanced diseases Cetuximab and panitumumab- directed against EGFR, preventing growth and proliferation of tumor cells o Side effect- acne-like rash Bevacizumab- against VEGF, antiangiogenesis agent o Combination with irinotectan containing combinations o Side effect- hypertension, thromboembolic events, proteinuria Partial liver resection: solitary hepatic metastases without clinical or radiographic evidence of additional tumor involvement 5-FU + folinic acid (leucovorin): for 6 months after resection of tumor in px with stage 3 disease leads to 40% decrease in recurrence rates and 30% improvement in survival FOLFOX: reduced recurrence 5-FU + folinic acid (leucovorin)+ irinotectan: did not enhance outcome stage II tumors: adjuvant therapy not effective 5 FU + radiation therapy: reduces risk of recurrence and increases chance of cure in stage II and III