Open Access version via Utrecht University Repository
advertisement

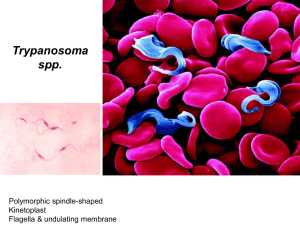
Human African trypanosomiasis in non-endemic areas: review on pathogenesis, clinical manifestation and therapy of cases published in the last 20 years Masters Thesis Stephanie Migchelsen, MSc student Biomedical Sciences, Universiteit Utrecht Supervisors: Dr Emily Adams- Koninklijk Instituut voor de Tropen- Biomedical Research Prof Dr IM Andy Hoepelman- Head, Dept of Internal medicine and Infection disease, UMC Utrecht Human African Trypanosomiasis (HAT), also known as African HAT, is caused by two subspecies of the flagellate protozoan Trypanosoma brucei. Trypanosomes are single-celled eukaryotic parasites and members of the Kinetoplastida order. These parasites are characterized by a kinetoplast, a granule containing DNA within the mitochondria and associated with movement of the flagella [1]. Other members of the kinetoplasts include the causative agents for Chagas disease (American trypanosomiasis), caused by T. cruzi [2] and the many species of leishmania (Leishmania donovani, L. viannia) that cause visceral and cutaneous leishmaniasis [3]. T. brucei is endemic to sub-Saharan Africa, where it is a major threat to public health in 36 countries [4]. Only two subspecies of T. brucei [5] are pathogenic to humans; T. b. gambiense causes a chronic form of HAT in West and Central Africa, while T. b. rhodesiense is the pathogenic agent for the more acute form of the disease and is endemic to Eastern Africa [1, 6]. The countries most at risk are Angola, Central African Republic, Chad, Congo, Democratic Republic of the Congo, and Sudan in Central Africa; Côte d’Ivoire and Guinea in West Africa and Uganda, Malawi and Tanzania in East Africa [7]. Fig 1. Map of HAT. From [4] 2 EPIDEMIOLOGY AND TRANSMISSION Up until the mid-2000s, there was a steady increase in the number of cases for a number of reasons: civil unrest had disrupted public health systems responsible for control programmes in endemic regions [5], changes in vegetation and climate, and migration of cattle herds [8]. Recently, the number of cases has gone down as vector control programmes are re-established in endemic areas [6, 7]. These programmes aim to reduce the number of HAT cases by controlling the tsetse fly population. Tsetse flies (Glossina) transmit trypanosomes and can be found in endemic foci in sub-Saharan Africa. It is estimated that Glossina fuscipes and the two related subspecies of G. palpalis and G. morsitans are responsible for over 90% of HAT cases [9]. Depending on the species, tsetse flies can be found along rivers and in swamps in western Africa and in the savannahs of eastern Africa [10]. These regions are roughly divided by the Great Rift Valley [6], although it has been reported that the two strains may have become sympatric in Uganda [11].Tsetse flies are not endemic outside of sub-Saharan Africa, making the disease relatively unknown in the developed world. Vector control is one of the most efficient methods of preventing HAT, both locally in villages and regionally across larger areas [9]. Blue and black targets [9] and traps baited with artificial scents, such as acetone [12], have been proven to be simpler and, some would argue, more cost-effective than the release of sterile males [13]. Fig 4. Tsetse trap. From [1] 3 With the re-establishment of vector control programmes in endemic areas, it should be possible to use these relatively low-tech and inexpensive methods to control and eliminate the tsetse flies. The WHO reports that approximately 17, 600 new cases are diagnosed each year, with a cumulative rate of 50,000- 70,000 cases. A totally of 60 million people remain at risk and 48,000 people die from HAT [14] (both forms combined), despite restored access to endemic areas, increased access to therapeutic drugs, the commitment of non-governmental organizations to combat the disease, and increased international awareness of the disease [15]. It is estimated that in endemic areas, HAT results in 1.78 million disability-adjusted life years (DALYs) lost [16]. With an average treatment cost of only US$17 per DALY averted, it is highly attractive [16] to treat cases of HAT, based on the rule of thumb that a cost of US$25 per DALY averted is highly attractive [17]. These figures do not take into account the estimated tens of thousands of cases that go unreported every year, which may be as high as 50,000 cases per year [16]. PATHOGENESIS The parasites are transmitted through the bites of infected tsetse flies [8], and undergo complex changes during their life cycle, which varies from the gut of the insect vector to the blood stream of the human host. The tsetse fly takes a blood meal from an infected vertebrate (animal or human) reservoir, ingesting bloodstream trypomastigotes. The parasites multiply in the fly’s midgut as metacyclics, transform then travel to the salivary glands where they multiply again. When the fly takes its next blood meal, the parasites are transferred and the new host is infected. The parasites once again transform into trypomastigotes and multiply by binary fission in body fluids such as blood, lymph and spinal fluid [18]. HAT can be identified in two stages, depending on whether parasites have passed crossed the blood-brain barrier (BBB) into the cerebrospinal fluid (CSF) [6]. After the parasites are inoculated into man, they proliferate at the infection site, causing an inflammatory nodule, also known as a trypanosomal chancre. This ulcer can be seen in approximately half of all patients infected with T. b. rhodesiense [19]; however it is rarely 4 seen in T. b. gambiense infections, possibly because most infections are detected after the chancre has disappeared [20]. Fig. 2 Typical trypanosomal chancre. From [21] Parasites then spread to the lymph nodes and reach the blood stream which marks the beginning of the hemolymphatic stage [5]. Up to 50% of European patients will develop a rash on the torso and most patients will have swollen, palpable lymph nodes [22]. The patient suffers from fever, headache, pruritus and generalized edema and malaise. Patients suffering from west HAT will show generalized lymphadenopathy, usually on the back of the neck, a condition known as Winterbottom’s sign. Parasites can, at this stage, be microscopically detected in blood, lymph and aspirates. 5 Fig 3. Trypomastigotes (“stumpy form”) among blood cells. From [5] Signs and symptoms may subside after the acute first stage. In the meningoencephalitic stage, parasites enter into the organs, including the CNS [5, 23]. Disease stage can be determined by the presence of trypanosomes in the CSF. As this requires a lumbar puncture, this is usually performed after an initial dose of suramin, to reduce the possibility of contaminating the CSF samples with trypomastigotes from the blood stream [24]. The trypanosomes cross the blood-brain barrier near intracellular junctions and is an active process [25].This process is quite rapid in T. b. rhodesiense infections, taking only a few weeks, but lasting a few months or even years in T. b. gambiense infection. As the disease progresses, the classical signs of HAT arise [26]: severe headaches, a disruption of the circadian rhythm, with night time insomnia and daytime somnolence, altered mental functions and personality changes may arise while generalised meningoencephalitis may lead to coma and death [8]. Other symptoms include anorexia, altered endocrine functions [27], demyelination and leukoencephalitis are also typical [28]. 6 DIAGNOSIS Parasite numbers of less than 100 trypanosomes/mL can be difficult to detect with microscopy alone [20]. Concentration methods such as microhematocrit centrifugation [29], quantitative buffy-coat analysis [30-32] or mini-anion exchange columns [33] can be used to concentrate the parasites for easier microscopic detection. In West Africa, many endemic screening programmes rely on the card-agglutination test for trypanosomiasis (CATT), which tests the agglutination of trypanosomes in the presence of specific antibodies, is a sensitive assay to detect T. b. gambiense antibodies in serum [34, 35]. T. b. gambiense antibodies can also be detected by ELISA or immunofluorescence [36-38] although these are not the most practical methods to be used in the field due to their energy and reagent requirements. CATT is not effective for detecting T. b. rhodesiense infections and microscopic visualization should always be performed to confirm a positive test. Molecular techniques, such as PCR, have been developed and evaluated but have yet to be adopted in the field, due to the need for trained technicians, the proper equipment, a constant source of power, and the required storage methods for the supplies [1, 8]. Furthermore, the tests must also be standardized and validated in a clinical setting [20]. Some researchers report prolonged positivity after successful treatment, which could be misinterpreted as a continued infection [39, 40]. Recent innovations such as a with a novel loop-mediated amplification (LAMP) have led to the development of techniques for the detection of trypanosomes and may prove to be a useful alternative to PCR [41]. LAMP has no need for a thermocycler, with the whole reaction taking place between 60-65ºC, have a high specificity thanks to a complex set of primers and the products are easily detectable using fluorescent dyes and UV light [41]. Trypanosomes are encapsulated in a variant surface glycoprotein (VSG) that protects the parasite from lytic factors in plasma [42]. After infection, the VSG is recognized by the host’s immune system, which leads nonspecific B-cell activation and to the production of IgM and IgG antibodies to neutralise the parasites in an attempt to decrease parasitemia [43]. A small percentage of trypanosomes, however, will have different surface coats and will escape detection, continuing to proliferate until new coat-specific antibodies are produced by the host’s immune system. This is termed “antigenic variation”. It is suspected that there are over 2000 VSG genes, 7 including pseudogenes, although only one gene is expressed at a time [44, 45]. This high degree of antigenic variation means that a vaccine will be extremely difficult to produce [46], and that a high level of IgM can usually be detected in the patient. This elevated measurement is considered a good indication of trypanosomal infection [28]. TREATMENT There are only four licensed drugs for the treatment of HAT [47]. Pentamidine and suramin are available to treat the disease before parasites invade the CNS. Pentamidine is the recommended drug for treatment of firststage west HAT. It is administered intramuscularly for one week and is generally well-tolerated [48]. Complications that arise from intramuscular injections include pain and swelling at the injection site, abdominal pain or gastrointestinal problems [49]. Suramin is recommended for treatment against first-stage west HAT. Nephrotoxocity, peripheral neuropathy and thrombocytopenia are frequent but mild side effects and can easily be treated. Because hypersensitivity is also quite common, it is recommended that a low test dose be administered [50] prior to beginning the rather long (30 days) and complex drug course [51]. To fully treat trypanosomal infection in the CNS, the drug must be able to cross the blood-brain barrier [8]; two such drugs are recommended. For treatment of second-stage HAT, melarsoprol remains the most widely-used drug; it is the only drug available to treat rhodesiense-caused HAT, is the most economical but requires a lengthy and complex treatment regimen, and can be highly toxic. This organoarsenic compound causes frequent adverse reactions that can be quite severe and even life-threatening. Post-treatment reactive encephalopathy (PTRE) occurs in 20% of all patients receiving melarsoprol and two to 12% of those receiving treatment die as a result of complications [52, 53]. Patients must be carefully monitored during the course of treatment and diazepam or dexamethasone are recommended to manage the encephalopathy [49]. The onset of headaches and fever can be indicative of possible complications [54]. The cause of PTRE remains unknown, although many hypotheses exist. These include: (i) sub-curative chemotherapy; (ii) immune complex 8 deposition; (iii) aberrant immune response to glial cell-associated antigens; (iv) autoimmune mechanisms; and (v) arsenical toxicity [55-59]. Eflornithine is the most recent of the treatments against African trypanosomiasis [14]. It has a much lower mortality than melarsoprol and therefore it is recommended as the drug of choice to treat second-stage gambiense-disease [60-62]. It is however, a very expensive drug and its prescription and use may underline the disparity in access to required pharmaceuticals that exists between first world and developing countries [63]. T. b. rhodesiense shows an innate reduced susceptibility against eflornithine and thus treatment with melarsoprol is recommended [64]. Treatment with eflornithine requires 2 weeks of injections repeated 4 times per day; bacterial infection at the site of the catheter can lead to sepsis but can easily be prevented with proper care [20]. Other potential side effects include anemia, gastrointestinal symptoms and convulsions [65]. Recently the Drugs for Neglected Diseases initiative (DNDi)recommended a combined nifurtimox-eflornithine treatment combining 7 days eflornithine (2 infusions per day) followed by 10 days of nifurtimox taken as an oral dose [14] thereby reducing the number of injections. Trials are on-going to make more efficient use of existing drugs; combination therapies with known trypanocides [66] as well as shorter treatment regimens of eflornithine and melarsoprol are being tested [67, 68]. Shorter drug regimens should translate to reduced admission times and greater patient adherence. For optimal treatment, skilled medical personnel are required, preferably ones familiar with the possible complications and treatment of HAT. While medical staff experience in dealing with HAT may be difficult to come by in non-endemic areas, patients are more likely to receive a more thorough treatment, including proper nutrition and complete monitoring [1]. Due to the fact that HAT is classified as a neglected tropical disease (NTD) [7, 69], there is little incentive for pharmaceuticals to invest in research, development or production of new anti-trypanosomal compounds. Those most in need of the drug are not able to pay for treatment and thus there is little financial enticement to produce these drugs. Recently the WHO, along with several international and non-governmental organizations [70-72] convinced Aventis, the pharmaceutical company that manufactures these drugs, to guarantee a gratis production 9 of pentamidine, melarsoprol and eflornithine [73], as well as support for research into new drugs and other means of control. Storage and transport of the drugs is to be overseen by Médecins Sans Frontières (MSF). Bayer has also agreed to provide gratis production of suramin and also to continue production of nifurtimox. Long-term availability of all trypanocides is still uncertain [21]. Cases of HAT are found only where tsetse flies are located, however some travelers have been reported as being HAT positive. Here, the non-endemic cases of HAT are reported as well as their frequency and outcome in an effort to raise awareness about this NTD. The effects of HAT are wide reaching, not only in Africa where it is an endemic disease, but also in non-endemic, developed areas. CLINICAL CASES FOUND IN LITERATURE A recent search of PubMed and ProMED Mail resulted in 59 reported cases of human African trypanosomiasis. The search was limited to the past 20 years (2010-1990). Many articles published before 1990 proved difficult to obtain through an academic journal subscription. ProMED Mail archives can be searched dating back to 1994, when it was founded; a search for “trypanosomiasis” returned 184 reports. In total, 8 cases were reported only on ProMED which were not encountered via the PubMed search. Many of these cases were also reported on TropNetEurop (http://www.tropnet.net/special_reports/tryps_ex_serengeti.pdf), a European surveillance network for imported infectious diseases. The other 51 cases were found through a PubMed search (search terms: “trypanosoma OR HAT OR African trypanosomiasis NOT Chagas NOT animal NOT reservoir”) and through a bibliographic search of articles. While it is apparent that not all cases will have been reported and/or published, we are confident that a large number of non-endemic cases have been included in this review. Migration, tourism, and military presence in areas at risk may lead to an increase in the number of cases seen in non-endemic areas [22]. For this reason, it 10 is important that clinicians and physicians be made aware of the risk of HAT, a disease that is fatal if left untreated. WEST AFRICAN TRYPANOSOMIASIS Fourteen cases of west HAT were detected in a literature search (Table 1). All the cases were either immigrants from endemic regions who had migrated to Europe or North America [74-77], or cases of ex-patriots who had been stationed in endemic regions [78-83]. Three reported cases did not specify the reason for exposure [8385]. All of the cases encountered were diagnosed after a significant amount of time had passed after the initial infection, which is typical for the chronic form of HAT as symptoms may subside while the immune system reduces the parasitic load [5]. Of the 14 cases in this review, 8 cases were diagnosed in the first stage and 6 were diagnosed in the second stage. Some interesting points arise from a review of these cases. A New Zealand man who was posted in Nigeria and Gabon and was treated in the United Kingdom [78], but was initially diagnosed with Loa loa and Schistosomiasis and was treated; however splenomegaly, lymphadenopathy and elevated IgM levels persisted. Two months after his initial presentation at the hospital, he returned and trypanosomes were detected in a blood smear and lymph node aspirates. He was treated and cured with suramin and difluoromethylornithine. Individually, these parasitic infections are rarely seen in nonendemic regions; for one patient to be diagnosed with all three seems “most improbable” [78]. It is important to remember that travelers to endemic regions may be exposed to many possible parasitic infections. Although one disease may be diagnosed, physicians should consider other possible infections, especially if atypical symptoms are present. It has previously been mentioned that it can be difficult to obtain the required pharmaceuticals to treat a patient. Bisoffi et. al. (2005) reported a case in which eflornithine for stage 2 west HAT was obtained from the WHO but was subsequently delayed by 9 days while the drugs were held at Italian customs [85]. Luckily symptoms abated once eflornithine treatment was begun; lymphadenopathy resolved and a normal neurologic status were 11 reported after the treatment. The patient had reported feeling unwell for over 6 months before seeking treatment; while it is unlikely that a 9-day delay caused any significant damage, this case does highlight the importance of having timely access to the required pharmaceutical treatment. Had the patient been suffering from the more acute east HAT, it is likely that this 9-day delay could have had severe ramifications, including coma or even death. One common problem across most of the cases in non-endemic regions is an initial misdiagnosis. Western HAT is difficult to diagnose; its chronic nature is characterized by low parasitemia and may remain undiagnosed and unrecognized for years [22, 81]. Clinical signs and symptoms are generally non-specific. Many patients are initially diagnosed and/or presumptively treated for malaria [84-86], however as symptoms persist there is a need to reassess the diagnosis. Another possible misdiagnosis is Epstein-Barr virus (EBV). EBV presentation is variable and in cases wherein no trypanosomes are initially detected EBV can indeed seem like an appropriate diagnosis [80]. In one particular case [80], the patient was incorrectly diagnosed with EBV and the correct diagnosis of HAT wasn’t obtained until after emergency hospitalization 6 months later. Patients with west HAT have been reported to have antibodies against Toxoplasma gondii, Strongyloides stercoralis [76], Epstein-Barr virus, cytomegalovirus [74], Plasmodium fieldi, Plasmodium brasiliana and Borrelia burgdorferi [87], further complicating diagnosis and reinforcing the shortcomings of serological testing and emphasizing the need for better and more sensitive means of detection in blood and CSF [88, 89]. In only one reported case of west HAT was a trypanosomal ulcer, or chancre, noted [79]. This wound at the site of infection is more common in cases of east HAT rather than west HAT. In a review of trypanosomal cases between 1904 and 1963, Duggan and Hutchison reported that only 19 of 84 patients infected with T. b. gambiense had chancre [19], however no description of the lesions are included. Tatibouet et. al. (1982) reviewed 12 cases of west HAT recorded in France between 1967 and 1979 and reported that 42% exhibited chancres, describing them as circumscribed, red, indurated nodules between 4-10 cm in diameter [90]. Chancres appear much more often in patients suffering from East African trypanosomiasis, with lesions in 7012 90% of cases appearing 5 to 10 days after being bitten by the infected tsetse fly, at around the same time as fever and detectable parasitemia in the blood [19]. Of the fourteen published cases of west HAT in non-endemic regions, only 4 were regarding infected immigrants from endemic areas. Prior to 1990, 5 imported cases were seen in the United States, all African nationals living abroad [21]. Of the four cases since 1990, two were seen in the Netherlands [75, 77] and a third was seen in France [74] and the fourth was treated in Canada [76]. All were successfully treated although the correct diagnosis was sometimes difficult to come to. EAST AFRICAN TRYPANOSOMIASIS East African trypanosomiasis is distributed throughout eastern and south-eastern Africa, an area encompassing Tanzania, Uganda, Kenya, Zambia, Zimbabwe and Rwanda, among others, countries that are receiving an increasing number of tourists from the developed world [91]. HAT due to T. b. rhodesiense is most likely to be seen in travelers to national parks or game parks, where the ungulate animals so popular with safari tourists, serve as a reservoir for the parasite [83]. Unlike west HAT, east HAT is a much more acute form of the disease; death can occur in less than 2 weeks after being bitten by an infected tsetse fly [92]. The most typical signs are the trypanosomal chancre at the sight of infection and high grade fever. Substantial parasitemia can be detected in the blood. Incubation can be a little as 3 days, although is most typically between 6-10 days. Approximately 20,000- 40,000 people are currently infected with east HAT in Africa [93]. More foreigners travel to areas endemic for east HAT than west HAT and thus more are infected with east HAT and more cases are seen by physicians in non-endemic areas. Because east HAT is endemic to areas popular with tourists, an increase in the number of cases seen in travelers returning to non-endemic areas may serve as a warning of a potential outbreak in the region. Such was the case in 2001, when nine cases of HAT were detected in Europe through TropNetEurop, a sentinel surveillance network of clinics in Europe PROMEDMAIL [53, 94, 95]. Although prior to the early 1990s the number of tourists infected with HAT has been very low, the area is endemic for the disease. All nine patients 13 had traveled to Serengeti and Tarangire National Parks in Tanzania, among other destinations. Ranging in age from 27 to 68, all but one (from South Africa) were from Europe. All showed fever and most of the patients showed the trypanosomal chancre. Microscopy of thick and thin blood smears showed trypanosomes. Six patients were treated with suramin during the early stage, however 3 had multiorgan failure and showed signs of cerebral involvement; these patients were treated with either pentamidine or melarsoprol. Specific pharmaceuticals were difficult to obtain and were not chosen for the specific disease stage of the patient, but rather availability [53]. One patient, a 53-year-old Dutch woman succumbed to the disease [95, 96]. This patient was treated with a single dose of suramine in South Africa and after trypanosomes were detected in the CSF, melarsoprol treatment was begun. The patient continued with her travels through South Africa. She continued to suffer from headaches, fever and neurological deterioration. Five days after the last dose of melarsoprol, the patient became paralyzed, went into a coma and required artificial ventilation. She was repatriated from South Africa to the Netherlands and died approximately 4 months after the on-set of symptoms. Similar to the problems faced obtaining pentamidine and eflornithine to treat cases of T. b. gambiense HAT, many physicians face difficulties acquiring suramin or melarsoprol to treat their patients suffering from T. b. rhodesiense HAT. In cases of east HAT, it is imperative that patients be treated as soon as possible, due to the acute nature of the infection. Within a one-week period in 2000, two patients were admitted to the Hospital for Tropical Diseases in London [97, 98]. Two men returning from safaris in Zambia and Tanzania, within days of each other, had been bitten several times by mosquitoes and tsetse flies. Both suffered from diarrhea, vomiting and fever. Upon admission to hospitals, numerous trypomastigotes were detected in microscopic examination of blood smears. Neither patient had any CNS involvement; both were treated with suramin in London. Treatment was generally uncomplicated but significant effort went into obtaining the drugs. The drug was not available at the hospital’s pharmacy or from regional infectious or tropical disease units in the United Kingdom, France or Belgium. A small supply was finally obtained from the Liverpool School of Tropical Medicine which sufficed until a more 14 complete course was provided by the CDC. These cases highlight the need for physicians involved in the management of patients from tropical regions to have easy access to the drugs required to treat the potentially life-threatening diseases these patients may encounter [97]. Even with rapid treatment, patients can face complications. Melarsoprol is an arsenical trypanocide that is used only for stage 2 treatment because of its numerous side effects. It must be administered using a propylene glycol solvent; both the drug and the solvent are highly irritating [21]. Cutaneous irritations, fever, vomiting, diarrhea and polyneuropathy are all common however the most severe reaction is post-treatment reactive encephalopathy (PTRE) which is lethal in approximately 5% of patients. Arsenical encephalopathy can present with convulsions, cerebral edema, coma, or mental disturbances [52]. The mechanisms underlying this complication is still unknown [96]; HAT-related encephalopathy is characterized by multifocal lesions in the deep white matter [99]. MRIs can be a valuable tool in the management of encephalopathic HAT; treatment should continue if the scan shows features of trypanosomal encephalopathy rather than melarsoprol-induced encephalopathy [96, 99]. A 30-year-old man was admitted to the Institute of Tropical Medicine in France after an insect bite while on vacation in Rwanda left him with a severe headache and anorexia [99]. Examination showed hepatosplenomegaly, lymphadenopathy and purpura. Trypanosomes were detected in blood, marrow and CSF smears, confirming CNS involvement. An MRI was performed and revealed a thickening of the meninges. Treatment with prednisolone and melarsoprol were initiated but twitching and encephalopathy developed after the second course of melarsoprol. A CT scan indicated lesions of the internal capsules. As previously mentioned, neurological abnormalities during HAT can be due to brain infection by the parasites or posttherapeutic reactive encephalopathy (PTRE), as the parasites cross the blood-brain barrier or diffuse lesions as a result of hypoxia caused by an unknown mechanism [52], respectively. 15 In the United States, the Centre for Disease Control and Prevention (CDC) provides all the pharmaceuticals to treat HAT. As such, it maintains records of all HAT patients treated. The disease is virtually unknown in the United States [100]; only 14 cases were diagnosed and treated between 1968 and 1985 [101] and less than ten were reported during the two-decade period of this search [21, 24, 43, 102-104]. With such a centralized system for the distribution of medication, any trends in cases would hopefully become immediately apparent. CONCLUSIONS Here, the cases of east and west HAT in non-endemic areas have been reviewed. HAT, in both its forms, is rarely encountered in the developed world; without experience and expertise required there will continue to be difficulties in the diagnosis [22]. Physicians should be made aware of the disease and always consider HAT if their patient has traveled to an endemic area. The above cases are an important example of a tropical disease brought to a non-endemic country by immigration and tourism. Non-endemic cases encountered over the past 20 years have been imported largely due to North Americans and Europeans traveling to endemic areas for various reasons, such as military training or tourism in game parks; also, cases are observed in immigrants arriving from endemic areas, or in ex-patriots returning from postings abroad. Interestingly, the epidemiology of HAT seen in non-endemic areas is the opposite of the disease epidemiology seen in Africa: of the estimated 50,000- 70, 000 cases in endemic areas of Africa, more than 90% of these cases are due to T. b. gambiense [4], while the remaining cases are due to T. b. rhodesiense. According to the WHO (J Jannin, unpublished), approximately 20 cases (40%) of T. b. gambiense cases and 30 T. b. rhodesiense cases (60%) are diagnosed yearly outside of endemic areas [22].T. b. gambiense is most commonly seen in migrants arriving from and expatriates living in or returning from rural areas in western Africa, whereas T. b. rhodesiense is most often seen in travelers returning from safari in east African game parks [83]. In the cases presented below, we found approximately 25% of cases were due to west HAT and 75% were due to east HAT. In cases such as the above-mentioned 2001 cluster of HAT in European travelers [43], travelers from nonendemic countries may act as a sentinel or surveillance tool for tropical diseases. They tend to travel widely, 16 exposing themselves to may types of diseases. Most often they return home, to a medical system capable of rapid and definitive diagnosis, during the incubation period of the infectious agent, although the medical systems may not readily recognize the symptoms and may lack the diagnostic tools and drugs. In this manner, potential outbreaks can be detected and a warning can be sent to developing countries that might not otherwise be aware of the situation due to a lack of rapid diagnosis [105]. Information from this cluster of cases was passed on to the Tanzanian government to increase surveillance in the affected region, which lead to an increase in vector control programmes [53]. It is interesting to compare African trypanosomiasis to its South American counterpart, Chagas disease. Chagas disease is well known for being transmissible- both vertically from mother to child as well as through infected blood or organs [2]. Chagas disease is a silent disease; once it enters the indeterminate phase, the parasites lives asymptomatically in its human host, replicating in the blood and organs and only 20-30% of those infected will enter the chronic stage, characterized by involvement of the heart or digestive system [2]. Many of those infected are unaware of their situation [106]. As such, they may migrate to non-endemic regions, unknowingly bringing the disease with them. Vertical transmission of Chagas disease, from mother to infant, has been well documented in North America and Europe [106-111]. Studies have also shown that unknowing immigrants have already infected donated blood and organs that have resulted in other contracting the disease, sometimes leading to fatal cases of Chagas [2, 110, 112-114]. In December 2006, the Food and Drug Administration (FDA) licensed the first test to screen for T. cruzi infections in potential blood and organ donors [115], however studies have shown that the seroprevalence of T. cruzi in select donor populations may range between 0-0.48% [116]. Many countries specifically screen migrants from countries endemic for Chagas disease [106, 110, 115, 117]. Conversely, only one case each of transfusion-mediated HAT [118] and vertically-transmitted case of African trypanosomiasis [119] have been reported in literature. Without treatment, HAT is 100% fatal [20]. Those infected with the fast-acting, east HAT likely wouldn’t even be able to pass on the parasites via blood transfusion or organ transplant. And while west HAT can go undetected and diagnosed for months or even 17 years, it too is unlikely to be transmitted person-to-person due to the fragile life cycle of the parasite [120]. Given the low incidence of cases in North America and Europe, both from returning travelers and immigrants from endemic areas, as well as high fatality level if not treated, it is unlikely that HAT will ever pose a threat to the blood supply in the way that Chagas disease does. One salient feature of the cases encountered in this study is that few trends are apparent. The age of infected patients ranges from 9 years old [121] to 72 [82]. A number of cases presented in the United Kingdom, the Netherlands, the United States and France, but also in Germany, Italy, Australia, Switzerland, Norway, Canada, Sweden, Mexico and South Africa. Despite the occasional difficulty in obtaining medication, only 2 fatalities were reported: both middle-aged European women [53, 83, 92, 95, 96, 122]. Both were initially treated in Africa and later succumbed to the disease in the Netherlands; one was Dutch while the other was German. Meanwhile the death rate in Africa is approximately 48,000 [14]. Perhaps one reason to explain the high rate of survival in non-endemic patients is their general good health. The patients who were treated in the above cases were from developed nations where, one can assume, they had access to nutritious food and general health care; many treating physicians specifically noted that their patients were in excellent health other than the trypanosomal infection [21, 53, 83, 85, 97, 123]. Only one of the patients had a co-infection (with Schistosomiasis and Loiasis, both readily treatable) [78]; none suffered from tuberculosis, malaria or HIV/AIDS, unlike many trypanosomiasis patients in endemic regions [51, 89]. Hospitals and medical teams in developed nations are better able to provide a holistic treatment of the patient, with round-the-clock monitoring, no shortage of general medical supplies as well as access to drugs to treat complications that may arise. All diagnoses were based on symptoms and microscopic detection of parasites in blood and/or lymph and/or CSF. In only two cases was PCR used to confirm diagnosis [76, 124], showing that molecular or serological techniques have yet to replace classic parasitological techniques [6], both in Africa for those diagnosed abroad as well as in hospitals in non-endemic areas. This however, doesn’t decrease the need for more sensitive and rapid diagnostic methods. Any new methods should taken into account the challenges posed by working in the field in remote areas; new methods should not be limited to hospitals or clinics. 18 Before embarking on travel to endemic areas, travelers should be warned of possible risks, not only for trypanosomiasis but also for any other endemic diseases that they may encounter. General recommendations to prevent tsetse fly bites include wearing light-coloured clothing that fully covers arms and legs [43] as well as the use of personal insecticides, although cases have shown that tsetse flies may not be completely averse to insecticides [123]. Without a doubt, the most effective means for preventing trypanosomiasis, both in travelers and in those living in endemic areas, is the control and reduction of vectors and reservoirs. Significant success was had with rudimentary control methods in the mid 20th century [1]. With the civil unrest present in sub-Saharan Africa in the late 1990s, vector control programmes fell by the wayside and resurgence in cases was seen in Africa, as well as in travelers who returned to the area after political stability was re-established [53]. Such data should serve to remind that while Westerners are only occasionally at risk of trypanosomiasis, 60 million people remain at risk in Africa [7] and that further research must be undertaken to treat this disease. Knowing that simple methods such as traps and targets, have proven very effective, and continue to be effective, should spur further investment in the disease. Even relatively small amounts of funding are likely to have disproportionately large returns [6]. With increased funding, it should be possible to eradicate the disease by 2015, as predicted by the WHO [7]. This goal requires that not only are reservoirs and parasites controlled, but also that effective and economical drugs are made available to those most in need. ACKNOWLEDGEMENTS The author would like to thank Dr Emily Adams of the Koninklijk Instituut voor de Tropen- Biomedical Research, Amsterdam, the Netherlands and Prof Dr IM Andy Hoepelman, head of the Department of Internal medicine and Infectious disease UMC Utrecht, the Netherlands for their supervision and critical revision. Many thanks also to Joanne Brathwaite for critical revision of the manuscript. 19 Table 1. Reported non-endemic cases of west HAT. CSF= cerebrospinal fluid, T. b. g.= T. b. gambiense, S= suramin, E= eflornithine, M= melarsoprol, P= pentamidine Age Sex Nationality Country of exposure Year Clinical Features/symptoms Diagnosis Stage Sub species Treatment Ref Nigeria, Gabon 1991 lesion, rash, fever, lymphadenopathy, splenomegaly, elevated IgM, lymph, blood I T. b. g. S, difluoromethylornithine [78] Angola 1992 fever, insomnia, elevated IgG and IgM - II T. b. g. E [74] CSF blood blood II I I T. b. g. T. b. g. T. b. g. S,M E P [75] [84] [79] CSF, blood II T. b. g. E [80] blood I T. b. g. P [81] blood, CSF, PCR II T. b. g. E [76] blood, CSF II T. b. g. E [83, 85] blood I T. b. g. P, E [83, 85] blood, lymph I T. b. g. P [82, 83] blood I T. b. g. P blood I T. b. g. P [82, 83] [83, 125] 32 M young M New Zealander (ex-pat) French (immigrantAngola) 52 32 45 F M M Dutch (immigrant Cameroon) Italian (-) French (ex-pat) Cameroon Zaire Gabon 1995 1996 1999 - M French (ex-pat) Guinea 2000 53 M Guinea 2000 42 M French (ex-pat) Canadian (immigrantZaire) Zaire 2002 44 M Italian (-) Gabon 2005 54 F Italian (-) C. African Rep. 2005 37 M French (ex-pat) Gabon 2007 72 M French (ex-pat) Gabon 2007 50 M French (ex-pat) Gabon 2009 rash (neck, shoulders), elevated IgM fever, malaise lesion, fever, elevated IgM, weakness, sweats, vomiting, myalgia, weight loss, splenomegaly, fever, elevated IgG and IgM, lesion lesion, chills, weakness, fever, lymphadenopathy, hepatosplenomegaly, elevated IgG and IgM insomnia, anorexia, fatigue, headaches,lymphadeopathy, elevated IgM, fever fever, headache, weakness, anorexia, lymphadenopathy, hepatosplenomegaly fever, headache, insomnia, fatigue, splenomegaly fever, fatigue, anorexia, headache, insomnia, rash, lymphadenopathies pruritus, fever, weakness, anorexia, lymphadenopathy, elevated IgG and IgM fever, fatigue, lesion, lymphadenopathy 27 F Dutch (immigrantAngola) Angola 2009 fatigue, insomnia, anorexia, depression, coma 21 CSF II T. b. g. E [77] Table 2. Reported non-endemic cases of east HAT. CSF= cerebrospinal fluid, T. b. r.= T. b. rhodesiense, S= suramin, E= eflornithine, M= melarsoprol, P= pentamidine Age Sex Nationality Country of exposure Year - M Swiss (tourist) Rwanda 1990 - M Swiss (tourist) Rwanda 1990 1991 1994 Clinical Features/symptoms "clinical signs of sleeping sickness" "clinical signs of sleeping sickness" fever, lesion, lymphadenopathy, chills, sweat, anorexia, malaise, diarrhea meningoencephalitis 1994 49 - M M American (tourist) French (solider) Tanzania, Kenya, Rwanda Rwanda - M French (solider) Rwanda 57 30 41 54 49 47 51 M M M F M F M Mexico (tourist) French (tourist) American (tourist) American (tourist) Kenya Rwanda Tanzania Tanzania American (tourist) Tanzania German (tourist) Zambia, Zimbabwe, Tanzania British (tourist) Zambia Diagnosis Stage Sub species Treatment Ref blood, CSF II T. b. r. M [83, 126] blood I T. b. r. S [83, 126] I II T. b. r. T. b. r. P, S M [43] [127] major inflammatory syndrome blood CSF blood, medulla II T. b. r. M [127] 1996 fever, headache, lesion, hepatic dysfunction, respiratory distress blood, lesion exudate, CSF II T. b. r. P, M [128] 1997 headache, weight loss, fever, hepatosplenomegaly, lymphadenopathy blood, marrow, CSF II T. b. r. M [99] 1999 1999 weakness, headache, fever, chills, sweats, anorexia, lesion, lymphadenopathy, fever, sweats, chills, myalgia, blood, CSF blood II I T. b. r. T. b. r. S, M S [102] [24] 1999 malaise, drowsiness, insomnia, fever, chills, sweats, headache, myalgia, lesion blood I T. b. r. S [24] 2000 fever, insomnia, jaundice, lesion, lymphadenopathy, mucosal hemorrhage, splenomegaly, ascites blood, marrow I T. b. r. S [129] 2000 lesion, myalgia, diarrhea, fever, vomiting, headache, rigors, sweats, blood I T. b. r. S [97, 98] 22 30 30 M F 32 M British (tourist) Australian (tourist) - (treated in Antwerp) Kenya, Tanzania Tanzania 2000 2000 Tanzania 2001 lesion, fever, diarrhea, vomiting fever, rigor, headache fever, chancre, headache, jaundice, hepatosplenomegaly 33 M Italian (tourist) Tanzania 2002 30 M Italian (tourist) Tanzania 2002 fever, headache, nausea, vomiting, skin lesion, lymphadenopathy skin lesion, local edema, fever, mild jaundice, multiorgan failure, hepatomegaly American (tourist) Tanzania (Kenya, Zimbabwe) 2002 fever, lesion, headache, fatigue, myalgia, vomiting, rash, Tanzania Tanzania Tanzania 2002 2002 2002 Tanzania 2002 lesion, fever lesion, fever fever, renal failure, acidosis, jaundice Tanzania Tanzania Tanzania 2002 2002 2002 lesion, fever fever lesion, fever, headache 37 7 patients 44 41 M 68 M 27 60 55 F M F F M American/Canadian British (tourist) Swedish (tourist) South African (tourist) Norwegian (researcher) Dutch (tourist) Dutch (tourist) 2002 Dutch (tourist) Tanzania Kenya, Tanzania British (tourist) Tanzania 2004 lesion, fever, headache, intracerebral manifestations, coma, death fever, headache, myalgia, vertigo lesion, fever, dry cough, vomiting 2004 abdominal pain, fever, lesion, dry cough, vomiting, lymphadenopathy 53 F Dutch (tourist) 28 M 9 M 14 M British (tourist) Tanzania 2003 23 blood blood I I T. b. r. T. b. r. S P, S [97, 98] [131] blood I T. b. r. S [132] blood I T. b. r. P, S [53, 94] blood I T. b. r. P [53, 94] blood I T. b. r. S [21] - II I T. b. r. T. b. r. T. b. r. P S [11] [43] [43] - II T. b. r. M [43] blood blood I I I T. b. r. T. b. r. T. b. r. S S S [43] [43, [95] [43, 94] blood II T. b. r. S, M [53, 83, 95, 96] blood lesion aspirate I T. b. r. S [130] I T. b. r. S [121] blood, lesion aspirate I T. b. r. S [121] 26 M British (solider) Malawi 2006 62 F American (tourist) Africa 2006 insomnia, lethargy, vomiting, chancre, lymphadenopathy, fever, rigors fever, lesion, elevated IgM, rash 4 patients - Canadian, British, Australian Malawi 2007 thrombocytopenia, hallucinations 38 M British (tourist) Namibia, Mozambique, Malawi, South Africa 44 F German (tourist) Tanzania 2009 25 F Dutch (tourist) Tanzania 2009 61 M Polish (tourist) Uganda, Rwanda 2009 2007 i) fever, lymphadenopathy, hepatomegaly ii) somnolence, myalgia, headache, sweats iii) somnolence, headache, fevers, nerve palsy lesion, fever, myalgia, malaise, diarrhea, convulsions, death fever, lymphadenopathy, lesion, headache fever, multi-organ failure, asthenia, lesion, chills, jaundice, respiratory distress, hepatosplenomegaly, mucosal hemorrhage 24 blood I T. b. r. S [83, 123] blood II T. b. r. P, S, M [103, 104] blood I T. b. r. S [133] [124] blood, CSF II T. b. r. i) S, M ii) E iii) S, M, P blood II T. b. r. S, M [83, 122] blood I T. b. r. S [134] blood I T. b. r. P [135] BIBLIOGRAPHY 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. Barrett, M.P., et al., (2003) The trypanosomiases. Lancet. 362(9394): 1469-80. Schmunis, G.A. and Z.E. Yadon, (2009) Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop. Scarisbrick, J.J.C.P.L.W., J.; Moody, A.; Armstrong, M.; Lockwood,D.; Bryceson, A.; Vega-Lopez, F., (2006) Clinical features and diagnosis of 42 travellers with cutaneous leishmaniasis. Travel Medicine and Infectious Diseases. 4: 14-21. Simarro, P.P., J. Jannin, and P. Cattand, (2008) Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 5(2): e55. Brun, R., et al., (2009) Human African trypanosomiasis. Lancet. 375(9709): 148-59. Stich, A., P.M. Abel, and S. Krishna, (2002) Human African trypanosomiasis. BMJ. 325(7357): 203-6. -, (2009) Human African trypanosomiasis. [cited 2010 2 February]; Available from: http://www.who.int/trypanosomiasis_african/en/. Kennedy, P.G., (2006) Diagnostic and neuropathogenesis issues in human African trypanosomiasis. Int J Parasitol. 36(5): 505-12. Lindh, J.M., et al., (2009) Improving the cost-effectiveness of artificial visual baits for controlling the tsetse fly Glossina fuscipes fuscipes. PLoS Negl Trop Dis. 3(7): e474. Burri C, B.R., (2003) Human African Trypanosomiasis, in Manson's tropical diseases, Z.A. Cook GC, Editor. Elsevier: Edinburgh. 1303-23. Picozzi, K., et al., (2005) Sleeping sickness in Uganda: a thin line between two fatal diseases. BMJ. 331(7527): 1238-41. Okoth, J.O., (1991) Description of a mono-screen trap for Glossina fuscipes fuscipes Newstead in Uganda. Ann Trop Med Parasitol. 85(3): 309-14. Okoth, J.O., E.K. Kirumira, and R. Kapaata, (1991) A new approach to community participation in tsetse control in the Busoga sleeping sickness focus, Uganda. A preliminary report. Ann Trop Med Parasitol. 85(3): 315-22. -, (2009)Human African Trypanosomiasis- Current Treatments. Drugs for Neglected Diseases initiative. -, (2006)Human African trypanosomiasis (sleeping sickness): epidemiological update. Wkly Epidemiol Rec. 81(8): 71-80. Fevre, E.M., et al., (2008)The burden of human African trypanosomiasis. PLoS Negl Trop Dis. 2(12): e333. -, (1996) Investing in health research and development. Ad Hoc Committee on Health Research Relating to Future Intervention Options-WHO: Geneva. -, (2009) Trypanosomiasis, African. Centre for Disease Control and Prevention. Duggan, A.J. and M.P. Hutchinson,(1966) Sleeping sickness in Europeans: a review of 109 cases. J Trop Med Hyg. 69(6): 124-31. Chappuis, F., et al., (2005) Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev. 18(1): 133-46. Moore, A.C., E.T. Ryan, and M.A. Waldron, (2002) Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 20-2002. A 37-year-old man with fever, hepatosplenomegaly, and a cutaneous foot lesion after a trip to Africa. N Engl J Med. 346(26): 206976. Lejon, V., et al., (2003) The challenge of Trypanosoma brucei gambiense sleeping sickness diagnosis outside Africa. Lancet Infect Dis. 3(12): 804-8. Enanga, B., et al., (2002) Sleeping sickness and the brain. Cell Mol Life Sci. 59(5): 845-58. Sinha, A., et al., (1999) African trypanosomiasis in two travelers from the United States. Clin Infect Dis. 29(4): 840-4. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. Masocha, W., M.E. Rottenberg, and K. Kristensson, (2007) Migration of African trypanosomes across the blood-brain barrier. Physiol Behav. 92(1-2): 110-4. Buguet, A., et al., (2001) The duality of sleeping sickness: focusing on sleep. Sleep Med Rev. 5(2): 139153. Dumas M, B.S., (1999) Clinical aspects of human African trypanosomiasis, in Progress in human African trypanosomiasis, sleeping sickness, B.B. Dumas M, Buguet A, Editor, Springer: Paris 215-33. Louis, F.J., P. Buscher, and V. Lejon, (2001) [Diagnosis of human African trypanosomiasis in 2001]. Med Trop (Mars). 61(4-5): 340-6. Woo, P.T., (1970) The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 27(4): 384-6. Truc, P., et al., (1998) Parasitological diagnosis of human African trypanosomiasis: a comparison of the OBC and miniature anion-exchange centrifugation techniques. Trans R Soc Trop Med Hyg. 92(3): 2889. Truc, P., et al., (1998) Simplification of the miniature anion-exchange centrifugation technique for the parasitological diagnosis of human African trypanosomiasis. Trans R Soc Trop Med Hyg. 92(5): 512. Ancelle, T., et al., (1997) [Detection of trypanosomes in blood by the Quantitative Buffy Coat (QBC) technique: experimental evaluation]. Med Trop (Mars). 57(3): 245-8. Lumsden, W.H., et al., (1979) Trypanosoma brucei: Miniature anion-exchange centrifugation technique for detection of low parasitaemias: Adaptation for field use. Trans R Soc Trop Med Hyg. 73(3): 312-7. Truc, P., et al., (2002) Evaluation of the micro-CATT, CATT/Trypanosoma brucei gambiense, and LATEX/T b gambiense methods for serodiagnosis and surveillance of human African trypanosomiasis in West and Central Africa. Bull World Health Organ. 80(11): 882-6. Magnus, E., T. Vervoort, and N. Van Meirvenne, (1978) A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T. B. gambiense trypanosomiasis. Ann Soc Belg Med Trop. 58(3): 169-76. Wery, M., et al., (1970) The diagnosis of human african trypanosomiasis (T. gambiense) by the use of the fluorescent antibody test. 2. First results of field application. Ann Soc Belges Med Trop Parasitol Mycol. 50(6): 711-30. Wery, M., S. Wery-Paskoff, and N. Van Wettere, (1970) The diagnosis of human African trypanosomiasis (T. gambiense) by the use of fluorescent antibody test. I. Standardization of an easy technique to be used in mass surveys. Ann Soc Belges Med Trop Parasitol Mycol. 50(5): 613-34. Magnus, E., et al., (1978) Use of freeze-dried trypanosomes in the indirect fluorescent antibody test for the serodiagnosis of sleeping sickness. Ann Soc Belg Med Trop. 58(2): 103-9. Kirchhoff, L.V., (1998) Use of a PCR assay for diagnosing African trypanosomiasis of the CNS: a case report. Cent Afr J Med. 44(5): 134-6. Solano, P., et al., (2002) Comparison of different DNA preparation protocols for PCR diagnosis of Human African Trypanosomosis in Cote d'Ivoire. Acta Trop. 82(3): 349-56. Kuboki, N., et al., (2003) Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol. 41(12): 5517-24. Taylor, J.E. and G. Rudenko, (2006) Switching trypanosome coats: what's in the wardrobe? Trends Genet. 22(11): 614-20. Panosian, C.B., et al., (1991) Fever, leukopenia, and a cutaneous lesion in a man who had recently traveled in Africa. Rev Infect Dis. 13(6): 1131-8. Marcello, L. and J.D. Barry, (2007) Analysis of the VSG gene silent archive in Trypanosoma brucei reveals that mosaic gene expression is prominent in antigenic variation and is favored by archive substructure. Genome Res. 17(9): 1344-52. 26 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. Marcello, L. and J.D. Barry, (2007) From silent genes to noisy populations-dialogue between the genotype and phenotypes of antigenic variation. J Eukaryot Microbiol. 54(1): 14-7. Stuart, K., et al., (2008) Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 118(4): 1301-10. Pepin, J. and F. Milord, (1994) The treatment of human African trypanosomiasis. Adv Parasitol. 33: 147. Bronner, U., et al., (1991) Pentamidine concentrations in plasma, whole blood and cerebrospinal fluid during treatment of Trypanosoma gambiense infection in Cote d'Ivoire. Trans R Soc Trop Med Hyg. 85(5): 608-11. -, (2007) Clinical Guidelines. Médecins Sans Frontières: Paris. Jordan, A., (2003) Tse-tse flies, in Medical insects and arachnids, R.C. RP Lane, Editor. Chapman and Hall: London. 333-88. -, (1986) Epidemiology and control of African trypanosomiasis. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 739: 1-127. Haller, L., et al., (1986) Clinical and pathological aspects of human African trypanosomiasis (T. b. gambiense) with particular reference to reactive arsenical encephalopathy. Am J Trop Med Hyg. 35(1): 94-9. Jelinek, T., et al., (2002) Cluster of African trypanosomiasis in travelers to Tanzanian national parks. Emerg Infect Dis. 8(6): 634-5. Blum, J., S. Nkunku, and C. Burri, (2001) Clinical description of encephalopathic syndromes and risk factors for their occurrence and outcome during melarsoprol treatment of human African trypanosomiasis. Trop Med Int Health. 6(5): 390-400. Poltera, A.A., (1980) Immunopathological and chemotherapeutic studies in experimental trypanosomiasis with special reference to the heart and brain. Trans R Soc Trop Med Hyg. 74(6): 70615. Lambert, P.H., M. Berney, and G. Kazyumba, (1981) Immune complexes in serum and in cerebrospinal fluid in African trypanosomiasis. Correlation with polyclonal B cell activation and with intracerebral immunoglobulin synthesis. J Clin Invest. 67(1): 77-85. Pentreath, V.W., (1989) Neurobiology of sleeping sickness. Parasitol Today. 5(7): 215-8. Pepin, J. and F. Milord, (1991) African trypanosomiasis and drug-induced encephalopathy: risk factors and pathogenesis. Trans R Soc Trop Med Hyg. 85(2): 222-4. Hunter, C.A., et al., (1992) Subcurative chemotherapy and fatal post-treatment reactive encephalopathies in African trypanosomiasis. Lancet. 339(8799): 956-8. Chappuis, F., et al., (2005) Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis. Clin Infect Dis. 41(5): 748-51. Balasegaram, M., et al., (2006) Melarsoprol versus eflornithine for treating late-stage Gambian trypanosomiasis in the Republic of the Congo. Bull World Health Organ. 84(10): 783-91. Checchi, F., et al., (2007) Nifurtimox plus Eflornithine for Late-Stage Sleeping Sickness in Uganda: A Case Series. PLoS Negl Trop Dis. 1(2): e64. Pecoul, B., et al., (1999) Access to essential drugs in poor countries: a lost battle? JAMA. 281(4): 3617. Iten, M., et al., (1997) Alterations in ornithine decarboxylase characteristics account for tolerance of Trypanosoma brucei rhodesiense to D,L-alpha-difluoromethylornithine. Antimicrob Agents Chemother. 41(9): 1922-5. Burri, C. and R. Brun, (2003) Eflornithine for the treatment of human African trypanosomiasis. Parasitol Res. 90 Supp 1: S49-52. Jennings, F.W., (1993) Combination chemotherapy of CNS trypanosomiasis. Acta Trop. 54(3-4): 20513. 27 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 91. Burri, C., et al., (2000) Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial. Lancet. 355(9213): 1419-25. Pepin, J., et al., (2000) Short-course eflornithine in Gambian trypanosomiasis: a multicentre randomized controlled trial. Bull World Health Organ. 78(11): 1284-95. -, (2009) Control of Neglected Tropical Diseases. Available from: http://www.who.int/neglected_diseases/en/. Trouiller, P., et al., (1999) Is orphan drug status beneficial to tropical disease control? Comparison of the American and future European orphan drug acts. Trop Med Int Health. 4(6): 412-20. Trouiller, P., et al., (2002) Drug development for neglected diseases: a deficient market and a publichealth policy failure. Lancet. 359(9324): 2188-94. Trouiller, P. and P.L. Olliaro, (1999) Drug development output: what proportion for tropical diseases? Lancet. 354(9173): 164. Etchegorry, M.G., et al., (2001) Availability and affordability of treatment for Human African Trypanosomiasis. Trop Med Int Health. 6(11): 957-9. Blanchot, I., et al., (1992) [Recurrent fever episodes in an African child: diagnostic difficulties of trypanosomiasis in France]. Pediatrie. 47(3): 179-83. Otte, J.A., et al., (1995) [African sleeping sickness in The Netherlands]. Ned Tijdschr Geneeskd. 139(41): 2100-4. Sahlas, D.J., et al., (2002) Clinical problem-solving. Out of Africa. N Engl J Med. 347(10): 749-53. Kager, P.A., et al., (2009) Magnetic resonance imaging findings in human African trypanosomiasis: a four-year follow-up study in a patient and review of the literature. Am J Trop Med Hyg. 80(6): 947-52. Scott, J.A., et al., (1991) Diagnosing multiple parasitic infections: trypanosomiasis, loiasis and schistosomiasis in a single case. Scand J Infect Dis. 23(6): 777-80. Iborra, C., et al., (1999) A traveler returning from Central Africa with fever and a skin lesion. Clin Infect Dis 28(3): 679-80. Raffenot, D., et al., (2000) [Infectious mononucleosis or sleeping sickness?]. Ann Biol Clin (Paris). 58(1): 94-6. Malvy, D., et al., (2001) [Human African trypanosomiasis from Trypanosoma brucei gambiense with inoculation chancre in a French expatriate]. Med Trop (Mars). 61(4-5): 323-7. Ezzedine, K., et al., (2007) Skin features accompanying imported human African trypanosomiasis: hemolymphatic Trypanosoma gambiense infection among two French expatriates with dermatologic manifestations. J Travel Med. 14(3): 192-6. Gautret, P., et al., (2009) Imported human African trypanosomiasis in Europe, 2005-2009. Euro Surveill. 14(36). Buyse, D., et al., (1996) [Sleeping sickness as an import pathology following a stay in Zaire]. Acta Clin Belg. 51(6): 409-11. Bisoffi, Z., et al., (2005) African trypanosomiasis gambiense, Italy. Emerg Infect Dis. 11(11): 1745-7. Taelman, H., et al., (1987) Difluoromethylornithine, an effective new treatment of Gambian trypanosomiasis. Results in five patients. Am J Med. 82(3 Spec No): 607-14. Damian, M.S., et al., (1994) [Polyneuritis and myositis in Trypanosoma gambiense infection]. Dtsch Med Wochenschr. 119(49): 1690-3. Lejon, V., et al., (2002) IgM quantification in the cerebrospinal fluid of sleeping sickness patients by a latex card agglutination test. Trop Med Int Health. 7(8): 685-92. -, (1998) Control and surveillance of African trypanosomiasis. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 881: I-VI, 1-114. Tatibouet, M.H., M. Gentilini, and G. Brucker, (1982) [Cutaneous lesions in human African trypanosomiasis]. Sem Hop. 58(40): 2318-24. -, (2009) Yearbook of tourism statistics. World Tourism Organization: Barcelona. 28 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. 107. 108. 109. 110. 111. 112. 113. 114. 115. Klaassen, B.S., YG., (2009) Een fataal geval van Oost-Afrikaanse slaapziekte in Tanzania. Tijdschrift voor Infectieziekten. 4(2): 61-5. -, (2006) Development and evaluation of new diagnostic tests for human African trypanosomiasis. Wkly Epidemiol Rec. 81(6): 59-60. Ripamonti, D., et al., (2002) African sleeping sickness in tourists returning from Tanzania: the first 2 Italian cases from a small outbreak among European travelers. Clin Infect Dis. 34(1): E18-22. Mendonca Melo, M., et al., (2002) [Three patients with African sleeping sickness following a visit to Tanzania]. Ned Tijdschr Geneeskd. 146(52): 2552-6. Braakman, H.M., et al., (2006) Lethal African trypanosomiasis in a traveler: MRI and neuropathology. Neurology. 66(7): 1094-6. Moore, D.A., et al., (2002) African trypanosomiasis in travelers returning to the United Kingdom. Emerg Infect Dis. 8(1): 74-6. Jones, J., (2000) African sleeping sickness returns to UK after four years. BMJ. 321(7270): 1177. Sabbah, P., et al., (1997) Human African trypanosomiasis: MRI. Neuroradiology. 39(10): 708-10. -, (2009) The U.S. commitment to global health : recommendations for the public and private sectors. Committee on the U.S. Commitment to Global Health. The National Academies Press: Washington D.C. Nieman, R.E., J.J. Kelly, and H.A. Waskin, (1989) Severe African trypanosomiasis with spurious hypoglycemia. J Infect Dis. 159(2): 360-2. Malesker, M.A., et al., (1999) Rhodesian trypanosomiasis in a splenectomized patient. Am J Trop Med Hyg. 61(3): 428-30. Uslan, D.Z., et al., (2006) A woman with fever and rash after African safari. Clin Infect Dis. 43(5): 609, 661-2. Kumar, N., et al., (2006) Melarsoprol-associated multifocal inflammatory CNS illness in African trypanosomiasis. Neurology. 66(7): 1120-1. Jelinek, T. and N. Muhlberger, (2005) Surveillance of imported diseases as a window to travel health risks. Infect Dis Clin North Am. 19(1): 1-13. Jackson, Y., et al., (2009) [Management of Chagas disease in Europe. Experiences and challenges in Spain, Switzerland and Italy]. Bull Soc Pathol Exot. 102(5): 326-9. Pehrson, P.O., M. Wahlgren, and E. Bengtsson, (1981) Asymptomatic congenital Chagas' disease in a 5-year-old child. Scand J Infect Dis. 13(4): 307-8. Jackson, Y., et al., (2009) Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis. 15(4): 601-3. Martinez de Tejada, B., et al., (2009) [Congenital Chagas disease in Geneva: diagnostic and clinical aspects]. Rev Med Suisse. 5(222): 091-2, 2094-6. Gascon, J., et al., (2007) [Diagnosis, management and treatment of chronic Chagas' heart disease in areas where Trypanosoma cruzi infection is not endemic]. Rev Esp Cardiol. 60(3): 285-93. Leiby, D.A., M.H. Fucci, and R.J. Stumpf, (1999) Trypanosoma cruzi in a low- to moderate-risk blood donor population: seroprevalence and possible congenital transmission. Transfusion. 39(3): 310-5. Dodd, R.Y. and D.A. Leiby, (2004) Emerging infectious threats to the blood supply. Annu Rev Med. 55: 191-207. Flores-Chavez, M., et al., (2008) Fatal congenital Chagas' disease in a non-endemic area: a case report. Cases J. 1(1): 302. Solves, P., et al., (2009) Chagas disease screening in cord blood donors. Transfusion. 49(5): 1023-4. Kirchhoff, L.V. and R.D. Pearson, (2007) The emergence of Chagas disease in the United States and Canada. Curr Infect Dis Rep. 9(5): 347-50. 29 116. 117. 118. 119. 120. 121. 122. 123. 124. 125. 126. 127. 128. 129. 130. 131. 132. 133. 134. 135. Leiby, D.A., et al., (2002) Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 42(5): 549-55. Lescure, F.X., et al., (2008) Chagas disease, France. Emerg Infect Dis. 14(4): 644-6. Hira, P.R. and S.F. Husein, (1979) Some transfusion-induced parasitic infections in Zambia. J Hyg Epidemiol Microbiol Immunol. 23(4): 436-44. Mbala, L., et al., (1996) Congenital African trypanosomiasis in a newborn child with current neurologic symptomatology. Trop Doct. 26(4): 186-7. De La Rocque, S., et al., (2004) Remote sensing and epidemiology: examples of applications for two vector-borne diseases. Comp Immunol Microbiol Infect Dis. 27(5): 331-41. Faust, S.N., et al., (2004) Sleeping sickness in brothers in London. Pediatr Infect Dis J. 23(9): 879-81. Klaassen, B. and Y. Smit, (2009) Een fataal geval van Oost-Afrikaanse slaapziekte in Tanzania. Tijdschrift voor Infectieziekten. 4(2): 61-5. Croft, A.M., et al., (2006) African trypanosomiasis in a British soldier. J R Army Med Corps. 152(3): 156-60. Checkley, A.M., et al., (2007) Human African trypanosomiasis: diagnosis, relapse and survival after severe melarsoprol-induced encephalopathy. Trans R Soc Trop Med Hyg. 101(5): 523-6. Hope-Rapp, E., et al., (2009) [Double trypanosomal chancre revealing West African trypanosomiasis in a Frenchman living in Gabon]. Ann Dermatol Venereol. 136(4): 341-5. Braendli, B., E. Dankwa, and T. Junghanss, (1990) [East African sleeping sickness (Trypanosoma rhodesiense infection) in 2 Swiss travelers to the tropics]. Schweiz Med Wochenschr. 120(37): 134852. Montmayeur, A., et al.,(1994) [The sleep-wake cycle during Trypanosoma brucei rhodesiense human African trypanosomiasis in 2 French parachutists]. Bull Soc Pathol Exot. 87(5): 368-71. Ponce-de-Leon, S., et al., (1996) Trypanosoma brucei rhodesiense infection imported to Mexico from a tourist resort in Kenya. Clin Infect Dis. 23(4): 847-8. Sanner, B.M., et al., (2000) Fulminant disease simulating bacterial sepsis with disseminated intravascular coagulation after a trip to East Africa. Intensive Care Med. 26(5): 646-7. Callens, S., et al., (2003) [Three patients with African sleeping sickness following a visit to Tanzania]. Ned Tijdschr Geneeskd. 147(12): 581; author reply 581. ProMED-mail.Trypanosomiasis, African- Australia ex Tanzania. ProMed-mail 2000; 7 Nov: 20001107.1943. <http://promedmail.org>. Accessed 2 March 2010. ProMED-mail.Trypanosomiasis, African- Europe ex Tanzania. ProMed-mail 2001; 16 Oct: 20011016.2542. <http://promedmail.org>. Accessed 2 March 2010. ProMED-mail.Trypanosomiasis, African- South Africa ex Malawi. ProMed-mail 2007; 12 Feb: 20070212.0532. <http://promedmail.org>. Accessed 2 March 2010. ProMED-mail.Trypanosomiasis, African- Netherlands ex Tanzania (SE). ProMed-mail 2009; 24 Jul: 20090724.2613. <http://promedmail.org>. Accessed 2 March 2010. ProMED-mail.Trypanosomiasis, African- Poland ex Uganda (Queen Elizabeth NP). ProMed-mail 2009; 10 Aug: 20090810.2844. <http://promedmail.org>. Accessed 2 March 2010. 30