Circulation - Springer Static Content Server
advertisement

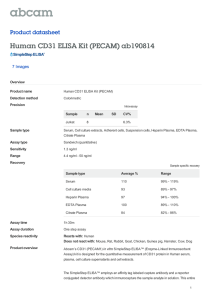
Flego et al Role of CD31 in Acute Coronary Syndromes SUPPLEMENTAL MATERIAL - TABLE OF CONTENTS Blood sampling page 1 Measurements of hs-CRP and cTnT page 1 Immunophenotypic analysis page 2 Phosphoflow analysis page 3 Cell co-cultures and immunofluorescence microscopy page 4 Supplemental Figure S1 page 5 Supplemental figure legend page 6 0 Flego et al Role of CD31 in Acute Coronary Syndromes 1 METHODS Blood sampling Venous blood samples were taken at the time of patient enrollment. In ACS, venous blood samples were collected within 24 hours from symptoms onset. Total and differential white blood cell counts (obtained with a Bayer H*3-hematology analyzer using automated cytochemistry in flow) and T-cell subset distribution were analyzed on fresh blood samples. Coded serum samples were stored at -70°C and analyzed for highsensitivity C-reactive protein (hs-CRP) in a single batch at the end of the study by laboratory staff unaware of the clinical data. In ACS patients, serum cTnT was determined at the time of hospital admission as routine measurement. All categorization and management of patients were independent from these results. Measurements of hs-CRP and cTnT hs-CRP was measured using a high-sensitivity latex-enhanced immunonephelometric assay (Latex/ BN II, Dade Behring, Marburg, Germany). The working range of the assay was 0.175 to 1100 mg/L, and the coefficient of variation was <5%. The median normal value for hs-CRP was 0.8 mg/L, with 90% of normal values <3 mg/L. cTnT was measured by the third generation cTnT assay on an Elecsys 2010 (Roche Diagnostics, Mannheim, Germany). The minimum detectable concentration was 0.01 ng/mL (99th percentile in healthy individuals). cTnT was <0.01 in all controls and SA patients. Flego et al Role of CD31 in Acute Coronary Syndromes 2 Immunophenotypic analysis T-cell subsets were assessed by flow-cytometry. Peripheral blood mononuclear cells (PBMC) were obtained from whole blood samples by standard gradient centrifugation over Ficoll-Hypaque (GE Healthcare Bio-Sciences, Piscataway, NJ). PBMCs were washed and resuspended in PBS containing 5% FBS. Then, PBMCs were incubated with fluorochrome-conjugated mAbs anti CD31 PE-Cy5 (clone WM-59 to detect the CD31 domains 2), (eBioscience, San Diego CA) and anti CD4-FITC and CD28 PE (Beckman Coulter, Brea, CA) to identify T-cells expressing CD31. Moreover, PBMCs were incubated with, anti CD31 PE-Cy5 (eBioscience, San Diego CA ), anti-CD4 FITC, antiCD45RA ECD, anti-CCR7 PE and anti-CD45RO PE-Cy7 to identified naïve T-Cells (Beckman Coulter, Brea, CA). At least 100,000 events were acquired. Non specific staining with isotype-matched control mAb was <1%; the intra- and inter-assay variability was <10%. FACS analysis was conducted with FC 500 (Beckman Coulter, Brea, CA) and the data were analyzed with Kaluza software (Beckman Coulter, Brea, CA). The expression of CD31 relative MFI was assessed dividing CD31 positive cells MFI with isotype control MFI. The CD31 positive cells were assessed as shown in Supplemental Figure 1. To assess the effect of CD31 in naïve T-cell activation, cells were purified from PBMCs by sorting with human Naïve CD4+ T Cell Isolation Kit II magnetic beads (Miltenyi Biotec, Auburn, CA). Purity of cell preparations was assessed by cytofluorometric staining. Cells were stimulated with anti-CD3/CD28 mAb (1 μg/ml each) and with or without anti-CD31 mAb (clone WM-59 functional grade purified) (1 μg/ml) (eBioscience, San Diego, CA ). After six days, cells were fixed and permeabilized in Flego et al Role of CD31 in Acute Coronary Syndromes 3 FIX/PERM buffer (eBioscience San Diego, CA) and stained with anti CD4-FITC (Beckman Coulter, Brea, CA), Ror-t-PE or T-bet-PE (eBioscience, San Diego, CA). Phosphoflow analysis PBMCs were starved overnight with RPMI 1% FCS at concentration of 1×106 /mL. Then, 1×106 PBMCs were stimulated with anti-CD3/CD28 mAb (or anti-CD3 alone for CD4+CD28nullT-cells) (1 μg/ml each) for TCR stimulation. To assess the biological role of CD31 on proximal TCR-signalling, anti-CD31 mAb (clone WM-59 functional grade purified) (1 μg/ml) (eBioscience, San Diego, CA ) was added to stimulated T-cells; goat anti-mouse IgG (BD bioscience, Mountain View, CA) was added for induction of crosslinking. Stimulation was started by transferring the cells to 37 °C water bath. After 5’, PBMCs were fixed and permeabilized in FIX/PERM buffer (eBioscience San Diego, CA) and stained for CD4-FITC, CD28 PE-Cy5 (Beckman Coulter, Brea CA) and Zap70-PE (pY-319 or pY-292) (BD bioscience, Mountain View CA).To assess the role of CD31 in TCR downstream signalling PBMCs were stimulated, fixed and permeabilized as describe above and stained with anti-CD4 FITC, anti-CD45 RA ECD, anti-CCR7 PECy5, anti-CD45RO PE-Cy7 (Beckman Coulter, Brea CA), anti-pERK-PE (pT202/pY204) or anti-p38-PE (pT180/pY182) (BD bioscience, Mountain View, CA). FACS analysis was performed as described above. The inhibitory effect of CD31 was calculated as: [(phospho-protein MFI stimulated αCD3/αCD28/αCD31 – phospho-protein MFI untreated) / (phospho-protein MFI stimulated αCD3/αCD28 – phospho-protein MFI untreated)] X 100. Flego et al Role of CD31 in Acute Coronary Syndromes 4 Cell co-cultures and immunofluorescence microscopy CD4+T-cells and monocytes for co-culture experiments were purified from PBMCs by sorting with CD4+ and CD14+ magnetic beads respectively (Miltenyi Biotec, Auburn, CA). Purity of cell preparations was assessed by cytofluorometric staining. CD14+ were cultured in RPMI 1640 medium supplemented with heat-inactivated 10% LPS-screened FCS, 1 mM sodium pyruvate, 0.1 mM non essential amino acids, 2 mM l-glutamine, 25 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Lonza, Basilea, CH) in the presence of GM-CSF (25ml) and IL-4 (25 ng/ml) (Miltenyi Biotec, Auburn, CA). After 6 days, immature monocyte derived dendritic cells (MDDCs) were washed and analyzed by flow-cytometry for the expression of surface markers CD11c, CD14, and CD31. MDDCs were used in the experiments if >90% were CD11c+ and <10% CD14+. Isolated CD4+T-cells were stained and incubated with Staphiloccocus Enterotoxin B (SEB) loaded MDDCs for 10’, fixed in IC Fixation buffer (eBioscience), blocked in PBS 20% FCS for 20’ and stained with Mouse-CD3 (Pierce, Rockford IL) and Rabbit-CD31 (Acris, Herford DE) antibodies labeled with alexafluor Rabbit anti-mouse 488 and Goat anti Rabbit 594 respectively (Invitrogen, Paisley UK). All images were captured by Leica DFC 420C and analyzed by LAS software (Leica Microsystem, Heerbrugg CH). Fold enrichment of molecules at the synapse was calculated as the ratio between the MFI of the contact region and the region outside the synapse after background subtractions. Data are presented as means of MFIs±SEM from a minimum of 20 cell-conjugates analyzed. Flego et al Role of CD31 in Acute Coronary Syndromes 5 Supplemental Figure S1 Flego et al Role of CD31 in Acute Coronary Syndromes 6 Supplemental figure legend Supplemental Figure S1. Gating strategy used to assess CD31 expression on T-cell subsets. The expression of CD31 was measured by dividing the MFI (mean fluorescence intensity) of CD31+ T-cells by MFI of isotype control. CD31+ cells were assessed with respect to isotype control. CD4+ = helper T-cells CD4+CD28- =CD28null T-cells CD4+CD45RA+CD45RO-CCR7+ = Naïve helper T-cells CD4+ CD45RA-CD45RO+ = “Primed” helper T-cells CD4+ CD45RA-CD45RO+ CCR7+ = Central memory helper T-cells CD4+ CD45RA-CD45RO+ CCR7- = Effector memory helper T-cells