U1 Obj 1-5 Chemistry13
advertisement

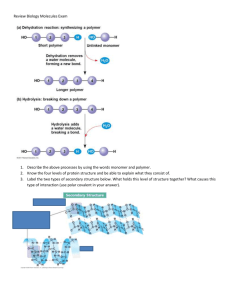
Unit 1 Objectives: The Chemistry of Life CHAPTER 1 OBJECTIVES: Exploring Life The Scope of Biology 1. What are the characteristics that biologists look for to determine "life"? 2. List and define the levels of biological organization. 3. Describe the interrelationship between the two major types of organisms in an ecosystem. 4. Develop a table listing the similarities and differences between the two major types of cells. Biological Systems 5. How can living things be "more than the sum of their parts"? 6. What are the benefits and drawbacks of reductionism? 7. In very simple terms, distinguish between positive and negative feedback. Biodiversity 8. Draw a concept map that demonstrates the relationship between the three domains and the six kingdoms. 9. Explain how there can be "unity in the diversity of life" and include the role of evolution by natural selection. Scientific Inquiry 10. 11. 12. 13. Explain how hypotheses are important in science. Contrast inductive and deductive reasoning. What are the key design aspects of a controlled experiment? Distinguish between the everyday meaning of the term ‘theory’ and its meaning to scientists. CHAPTER 2 OBJECTIVES The Chemical Context of Life Chemical Foundations 1. Define in as few words (<5) as possible the following terms: Matter, element, isotope, compound, atom, molecule Bonding 2. Explain the importance of electronegativity in the formation of polar and nonpolar covalent bonds. 3. Explain how ionic bonds form. 4. Why are hydrogen bonds so important? Page 1 CHAPTER 3 OBJECTIVES Water and the Fitness of the Environment The Properties of Water 1. Use your knowledge of chemistry (and the Chapter 2 Objectives) to explain the structure of a water molecule. 2. Develop a table to show examples of how the four emergent properties of water (rows) affect both plants and animals (2 columns). The Dissociation of Water 3. 4. 5. 6. Draw the equation for the dissociation of water and label the products. Draw the equation for the formation of hydronium. Describe the pH scale. Explain why buffers are so important in biological systems. CHAPTER 4 OBJECTIVES Carbon & the Molecular Diversity of Life The Importance of Carbon 1. Explain how carbon’s electron configuration accounts for its ability to form large, complex, and diverse organic molecules. 2. Describe how carbon skeletons may vary, and explain how this variation contributes to the diversity and complexity of organic molecules. 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer. Functional Groups 4. Using figure 4.10 as a guide, develop a table that includes the following info on the six functional groups: Group name Simple and structural formula Name of the compounds containing that group An example of a macromolecule (from Ch 5) that contains that group Page 2 CHAPTER 5 OBJECTIVES Structure & Function of Macromolecules The Principles of Polymers 1. Using glucose and fructose as the monomers and sucrose as the polymer, diagram the condensation (dehydration synthesis) reaction that builds sucrose and the hydrolysis reaction that breaks it down. Carbohydrates Serve as Fuel and Building Material 2. Define carbohydrate. Be sure to mention composing elements and common functional groups. 3. Describe the formation of a glycosidic linkage. 4. Distinguish between the glycosidic linkages found in starch and cellulose. Explain why the difference is biologically important. 5. Develop a table that shows the storage and structural form of carbs (rows) in plants and animals (columns). Lipids are a Diverse Group of Hydrophobic Molecules 6. Define lipid. Be sure to mention composing elements, building blocks and common functional groups. 7. Describe the biological importance of fats, phospholipids, and steroids. 8. Describe the formation of an ester linkage. 9. Distinguish between saturated and unsaturated fats. 10. Name the principal lipid storage molecules of plants and animals. Proteins have Many Structures and Many Functions 11. Define protein. Be sure to mention composing elements, building blocks and common functional groups. 12. Distinguish between a protein and a polypeptide. 13. Describe the formation of a peptide bond between two amino acids. 14. Draw the general structure of an amino acid and label the four major components/functional groups. 15. Describe the four levels of protein structure and identify what types of bonds are present at each level 16. List four conditions under which proteins may be denatured. Nucleic Acids Store and Transmit Hereditary Information 17. List the major components of a nucleotide, and describe how these monomers are linked to form a nucleic acid. 18. Distinguish between: a. pyrimidine and purine b. nucleotide and nucleoside c. ribose and deoxyribose 19. Briefly describe the three-dimensional structure of DNA. Page 3