APPENDICES FOR ONLINE PUBLICATION ONLY Appendix A (1
advertisement

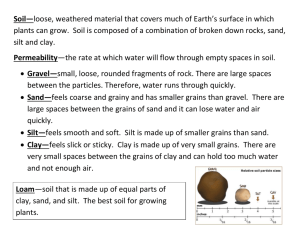
APPENDICES FOR ONLINE PUBLICATION ONLY Appendix A (1-3). Photos of the Cave Creek archaeological complex, including landscape patch types used in this study. 1. Plots located between silt fields (lower right) and on the floodwater-irrigated silt fields (upper right), both located on the western bank of Cave Creek. Woody plant diversity and biovolume of Larrea tridentata is significantly higher between fields compared to on fields (Briggs and others 2006). To the left is an aerial photo of the silt fields, taken after a wet winter in March 2005 (photographer H. Schaafsma is facing north, flying with pilot Joe Vogel). Note the higher elevation eastern bank (Pleistocene-aged substrate) where alignments are located compared to lower elevation western bank (Holocene-aged substrates) where floodwater-irrigated silt fields are located. 1 2. Plots located between rock alignments (left upper and left lower panels) and behind rock alignments (right upper and right lower panels), both located on the eastern bank of Cave Creek. White arrows point to the rock alignment. Photo credit: D. Nakase. 2 3. Grid gardens used for Hohokam dryland farming (not used in this study), located on the western bank of Cave Creek just north of the irrigated silt fields. Inset photo: Rock art found at the site. Photo credit: H. Schaafsma. 3 Appendix B. Soil profile textural descriptions from locations near our study site, including prehistoric floodwater irrigated fields, between silt field locations, and rain-fed rock alignment areas used for runoff farming. Soil profiles within the agricultural floodwater-irrigated fields (‘silt fields’). Huckleberry (2011) excavated a soil pit within a Hohokam floodwater-irrigated field located just to the south of our study area along Cave Creek (the southern-most silt field shown in Figure 1). Soil texture within this silt field is uniformly silt loam or loam down to the lowermost Ck horizon at 92 cm, after which textures reflect the sandy parent material (sandy loam or sand). Soil profiles within the desert areas between floodwater-irrigated agricultural fields (‘between silt fields’). The areas between the silt fields were not excavated in this study, but descriptions by Huckleberry (2011) and Schaafsma and Briggs (2007) suggest that these locations are dominated by uniformly sandy soils. NRCS maps classify these soils as part of the Antho-Carrizo-Maripo complex (Torrifluvents) composed of a shallow A horizon over a C horizon, composed of approximately 67-90% sand and 10% or less clay from the surface until 76 cm depth (NRCS 2013). These soil characteristics are consistent with our surface soil analyses in the ‘between silt field’ patch type (mostly sand, with ~10% clay). Another soil, the Mohall clay loams, also exist on the eastern side of Cave Creek between silt fields, but these soils are located further from the channel and have surface textures with approximately 30% clay. Soil profiles of the Carefree series, which include soils within the ‘rock alignment area’. Huckleberry (2011) excavated a pit near archaeological sites AZ T:4:410 (ASM) and AZ T:4:411 (ASM) on Carefree series soils located around 6 km to the SW of our site along a tributary of Cave Creek. These soils are similar to our ‘alignment’ soils on the Pleistocene-aged surface to the east of Cave Creek. At this site, Huckleberry found silt loam horizons (0-8 cm) overlying a series of clay loam Bt horizons extending from 8 to 65 cm, followed by a sandy clay loam at depth (>65 cm). The NRCS description of the Carefree series shows similar patterns, including a shallow A loam or clay loam horizon (0-3 cm) changing to a Bt below 3 cm with about 45% clay and about 25% sand. Huckleberry, G. 2011. Geoarchaeological investigation of the Apache Wash-Cave Creek Area and Assessment of Hohokam Sediment Capture and Soil Modification, Maricopa County, Arizona. Logan Simpson Design Inc, Tempe, AZ. NRCS. 2013. Soil Survey Geographic (SSURGO) Database. Soil Survey Staff. Natural Resources Conservation Service, United States Department of Agriculture. Schaafsma, H. and J. M. Briggs. 2007. Hohokam Field Building: Silt fields in the northern Phoenix basin. Kiva 72:443-469. 4 Appendix C. Methods for soil and plant community composition analyses Pollen analyses To complement the pollen database derived from samples on the western, floodwaterirrigated side of Cave Creek, we collected soil samples for pollen analysis at 2-5 cm depth from the eastern side of the creek where anthropogenic rock alignments were located (N = 7 soil samples for pollen analysis each from between and behind constructed rock alignments; total N = 14 soil samples). Results from these analyses showed that pollen assemblages did not differ in soil collected behind alignments and between alignments (Smith 2009). Field soil samples from archaeological contexts preserve a low level of crop pollen, making it difficult to recover and the results difficult to interpret (Fish 1995). For example, maize pollen is not always recovered in samples from modern fields where this crop is known to have been grown (Fish 1984). Thus, the lack of maize pollen in soil does not preclude the interpretation that these features were used for cultivation of this resource (Sandweiss 2007). Nevertheless, it is likely that these alignments were used less intensively than fields used for floodwater farming, for either staple crops or potentially to ‘encourage’ native plants such as cholla (Cylindropuntia spp.), agave (Agave spp.), and a variety of native desert annuals – a number of which were domesticated, for example, Plantago spp.– that were used for food or other economic purposes (Bohrer and others 1969; Miksicek 1983; Fish 1984; Bohrer 1991; Doolittle 2000; Appendix D). Soil and plant tissue laboratory methods All soil properties and processes were determined on 2 mm sieved soils. Soil particle size was determined using the hydrometer method (100 mL of 50 g/L sodium hexametaphosphate in 40 g of soil), followed by sieving to 53 μm for sand content and calculating silt content by difference. Upon analysis with the modified pressure calcimeter method (Sherrod and others 2002), surface soils contained less than 1% carbonate and less than 5% organic matter; thus, these soil fractions were not removed prior to particle size analysis (Gee and Bauder 1986). Gravimetric soil moisture (%) was determined by drying 30 g of soil for 24 h at 105C. Waterholding capacity of the soil (WHC) was calculated as the water (%) held in 20 g soil after a 24 h drain time through a GF-A filter using soils from Spring 2008. Soil organic matter content (SOM; %) was estimated by the loss-on-ignition method as ash-free dry mass following combustion of oven-dried soils for 4 h at 550C. Total soil carbon and nitrogen were determined by combustion on a subset of soils (N = 4) following grinding using a CHN analyzer (PerkinElmer 2400 Series II CHNS/O Analyzer; Waltham, MA, USA). To determine pH and electrical conductivity (EC), slurries of 15 g soil with 30 mL deionized water were measured using a portable pH conductivity meter (VWR sympHony, Bristol, Connecticut, USA) and conductivity meter (Hach Co., Loveland, CO, USA). Ammonium (μg NH4+-N·g-1 dry soil) and nitrate + nitrite (summed as μg NO3--N·g-1 dry soil) concentrations were measured by first extracting 10 g of soil with 50 mL of 2M KCl for 1 h on an orbital shaker. Extracts were filtered through pre-leached Whatman #42 ashless filters, frozen, and later thawed and analyzed colorimetrically using a Lachat Quikchem 8000 autoanalyzer (Hach Co, Loveland CO, USA). Potential rates of net N mineralization and net nitrification were assessed by incubating 10 g of soil at 60% WHC in the dark at 20°C for 10 days, followed by extraction with 2M KCl and colorimetric analysis as 5 described above. Rates of potential net N mineralization and net nitrification were calculated as the difference in the sum of NH4+ and NO3-, or NO3- alone, respectively, before and after incubation divided by the number of incubation days (μg N g-1 d-1). Extractable phosphate concentration (μg PO43-P·g-1 dry soil) was measured by first extracting 2 g of soil with 40 mL of 0.5M NaHCO3 after shaking for 1 h. Extracts were filtered through pre-leached Whatman #42 ashless filters, were frozen, and thawed samples were analyzed colorimetrically with a BranLuebbe Traacs 800 Autoanalyzer (SEAL Analytical Inc. Mequon, WI, USA). Effective cation exchange capacity (ECEC) was determined once per site and landscape patch type. ECEC was measured on soils collected in October 2008 by extracting 10 g of soil for 12 h with 50 mL of 1 M ammonium acetate adjusted to pH 7, filtering extracts through pre-leached Whatman no. 42 filters, and then freezing extracts prior to analysis for potassium (K+), calcium (Ca2+), magnesium (Mg2+), and sodium (Na+) with an ICP-OES (Thermo iCAP 6300, Waltham, MA, USA). ECEC was calculated as (cmol element kg-1 soil) = exchangeable K+ + exchangeable Ca2+ + exchangeable Mg 2+ + exchangeable Na+ (Sumner and Miller 1996). Bulk density of the 0-5 cm depth of surface soils was determined from two, homogenized 6.25 cm diameter soil cores from 5 of the 12 replicates of each of the 4 feature types (20 cores total). Soil weight and volume were corrected for greater than 2 mm coarse fragments. One core (of 20) with greater than 10% coarse fragments was excluded from the analysis due to inaccuracies in the coring method. Bulk densities of the remaining samples were not significantly different from one another among the finer-textured soils (eastern side of Cave Creek and silt fields, N = 15, range 1.3-2.0 g/cm3, Tukey HSD, p = 0.74), but densities of the fine-textured soils were significantly lower than the more coarse-textured soils located in between the silt fields (N = 4, range 1.9-2.3 g/cm3). Thus, an average BD of 1.75 g cm-3 and 2.14 g cm-3 for the finer-textured soils and coarse textured soils between silt fields, respectively, was used to convert nutrient concentrations (µg N or P g-1 dry soil) to pools (µg N or P cm-2) and fluxes (µg N cm-2 day-1). Plant tissue concentrations of C, N, and P. Aboveground tissue of Plantago ovata was dried at 60°C at ASU, and was coarsely ground with mortar and pestle then ball milled to a fine powder. Tissue C and N concentrations were determined using a Perkin-Elmer 2400 Series II CHNS/O Analyzer (Waltham, MA, USA). Tissue P concentration was determined using the persulfate digestion and ascorbic acid method followed by colorimetry (Schade and others 2003). Absorbance was determined with a Thermo Electron Corporation Genesys 10uv spectrometer (Madison, Wisconsin, USA) at 880 nm. Methods for PCA, NMDS, ANOSIM, and SIMPER analyses PCA: To assess the effect of landscape patch type on combined soil properties, factor analysis (PCA) was applied to the correlation matrix of soil physical variables as well as those variables associated closely with biotic processes (total extractable inorganic N and P pools, and potential rates of net N mineralization assessed during Spring 2008; see variables in Table 2). Factors were extracted using the principal components method (PCA) followed by an orthogonal varimax rotation. Components with eigenvalues above 1 were retained in the analysis. In interpreting the rotated factor pattern, a variable was said to load on a given component if the factor loading was above 0.6 or below -0.6. MANOVA was used to determine the importance of landscape patch type in the composition of the significant principal factors. Post-hoc Tukey (or 6 Games-Howell) tests were conducted on the univariate solutions to determine differences between landscape features (α = 0.05/2 factors = 0.025). NMDS: We also explored differences in community composition among feature types using non-metric multidimensional scaling (NMDS) ordination in PC-ORD using the Sorensen (Bray-Curtis) distance metric on square-root transformed cover data with rare species removed (<5% in all sample plots). We first used a random starting configuration with a maximum of 6 axes, 250 runs with real data, and the same number of runs with randomized data followed by a Monte Carlo test of significance. We chose a 3 dimensional solution based on evaluation of stress values by dimension (McCune and Grace 2002, Peck 2010). We then ran the NMS procedure with 3 dimensions four additional times (250 runs of real data followed by 249 runs of randomized data) with orthogonal axis rotation and ended with a stable final run of 88 iterations and stress value of 12.1. After the analysis, we determined the fraction of variation represented by each axis by calculating a coefficient of determination (r2) from the relationship between the distances in the original, unreduced matrix to distances among the points in ordination space (McCune and Grace 2002). ANOSIM and SIMPER: ANOSIM tests were based on the same Bray-Curtis distances used in the NMDS analyses (square-root transformed cover data with rare species removed) followed by pairwise multiple comparisons tests corrected for the number of analyses (12 comparisons between four feature types; alpha = 0.008) using the software, PAST 1.94b (Hammer and others 2001). We followed the ANOSIM with a SIMPER analysis (Similarity Percentage; Clarke 1993) in PAST to identify which species were most responsible for dissimilarity between feature types. Bohrer, V. L., H. C. Cutler, and J. D. Sauer. 1969. Carbonized Plant Remains from Two Hohokam Sites, Arizona BB:13:41 and Arizona BB:13:50. Kiva 35:1-10. Bohrer, V. L. 1991. Recently Recognized Cultivated and Encouraged Plants among the Hohokam. Kiva 56:227-235. Clark, C. P., L. Aguila, M. Glass, B. G. Phillips, and V. Vargas. 1999. AZ U:1:11 (ASM): KCCF Towers Project, Maricopa County, Arizona. Archaeological Consulting Services, Tempe, AZ. Doolittle, W. E. 2000. Cultivated Landscapes of Native North America. Oxford University Press, New York. Fish, S. K. 1984. Agriculture and Subsistence Implications of the Salt-Gila Aqueduct Project Pollen Analysis. Pages 111-138 in L. S. Teague and P. L. Crown, editors. Hohokam Archaeology along the Salt-Gila Aqueduct Central Arizona Project Environmental and Subsistence. Cultural Resource Management Section, Arizona State Museum, Tucson. Fish, S. K. 1995. Archaeological Palynology of Gardens and Fields. Pages 44-69 in N. Frances and K. Gleason, editors. The Archaeology of Garden and Field. University of Pennsylvania Press, Philadelphia, PA. 7 Gee, G. and J. Bauder. 1986. Particle-size analysis. Pages 383-411 in A. Klute, editor. Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods. American Society of Agronomy/Soil Science Society of America, Madison, WI. Hammer, Ø., D. A. T. Harper, and P. D. Ryan. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4:1-9. McCune, B. and J. B. Grace. 2002. Analysis of ecological communities. MjM Software Design, Miksicek, C. H. 1983. Appendix B Plant Remains From Agricultural Features. Pages 605-620 in L. S. Teague and P. L. Crown, editors. Hohokam Archaeology Along the Salt-Gila Aqueduct Central Arizona Project. Cultural Resource Management Division, Arizona State Museum, University of Arizona, Tucson. Peck, J. E. 2010. Multivariate analysis for community ecologists: Step-by-step using PC-ORD. MjM Software Design, Gleneden Beach, Oregon, USA. Sandweiss, D. H. 2007. Small is big: The microfossil perspective on human–plant interaction. Proceedings of the National Academy of Sciences of the United States of America 104:3021–3022. Schade, J. D., M. Kyle, S. E. Hobbie, W. F. Fagan, and J. J. Elser. 2003. Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecology Letters 6:96-101. Smith, S. J. 2009. Pollen analysis from natural and constructed pre-Columbian terraces along Cave Creek, Maricopa County, Arizona. Northern Arizona University, Flagstaff, AZ. Sumner, M. E. and W. P. Miller. 1996. Cation exchange capacity and exchange coefficients. Pages 1220-1223 in D. L. Sparks, A. L. Page, and P. A. Helmke, editors. Methods of Soil Analysis, Part 3, Chemical Methods. Soil Science Society of America,, Madison, Wisconsin, USA. 8 Appendix D. Percent Cover of Plant Species and Their Food/Economic Importance to Hohokam People across Landscape Patch Types within the Cave Creek Archaeological Complex a Food or economic Between alignments % Cover Behind Alignments % Cover Constancy Between silt fields % Cover Constancy Constancy % Cover Silt fields Constancy Transformation/ Mean SE % quadrats Sig. Mean SE % quadrats Sig. Mean SE % quadrats Sig. Mean SE % quadrats Sig. test p value 6.18 3.49 91 a 7.32 2.50 64 a 32.95 4.55 100 b 1.36 0.70 27 a sqrt/ANOVA <0.001 1, 2 24.32 4.05 100 a 26.82 6.26 82 a 3.64 0.70 73 b 30.00 5.17 100 a cube rt/ANOVA <0.001 Erodium texanum 1 22.05 7.63 82 a 3.64 2.11 27 b 0.00 0.00 0 b 17.95 4.23 91 a none/Kruskal-Wallis <0.001 Erodium cicutarium 1 Yes 26.41 7.09 100 a 33.00 9.23 91 a 22.55 8.62 82 a 8.18 3.23 82 a sqrt/ANOVA 0.11 2 27.27 8.33 100 a 31.82 8.18 91 a 9.55 1.90 100 a 11.41 2.14 100 a cube rt/ANOVA 0.14 Species importance? Pectocarya recurvata Plantago ovata Schismus spp. Yes Yes No Astragalus didymocarpus 7.55 2.01 91 10.09 3.44 91 0.50 0.45 18 6.59 6.11 18 Lepidium lasiocarpum 1 5.00 2.42 73 6.23 2.27 73 3.73 2.10 45 2.41 0.75 73 Amsinckia intermedia 2 0.00 0.00 0 1.68 1.58 27 9.64 6.70 36 2.05 1.61 18 Cryptantha sp.1 0.05 0.05 9 10.73 5.20 45 0.00 0.00 0 0.45 0.45 9 Plagiobothrys arizonicus 0.00 0.00 0 0.00 0.00 0 8.00 7.95 18 0.00 0.00 0 Astragalus nuttalianus 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 7.41 4.51 55 Orthocarpus purpurascens Yes Yes 2 0.00 0.00 0 0.00 0.00 0 7.05 3.69 36 0.00 0.00 0 Monolepis nuttalliana 1, 2 No 0.64 0.44 45 1.00 0.60 36 3.95 1.54 73 0.00 0.00 0 Larrea tridentata 1, 2 0.00 0.00 0 0.00 0.00 0 4.77 2.46 27 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 3.91 1.55 64 0.00 0.00 0 0.00 0.00 0 3.64 2.11 27 0.00 0.00 0 0.00 0.00 0 1.45 0.69 45 2.14 1.60 36 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 3.41 3.41 9 0.00 0.00 0 0.00 0.00 0 Guillenia lasiophylla 2.55 1.61 36 0.45 0.45 9 0.00 0.00 0 0.00 0.00 0 Herniaria cinerea 2.05 1.61 18 0.91 0.61 18 0.00 0.00 0 0.00 0.00 0 Yes Yes Loeflingia squarrosa Lasthenia californica 1 Yes Erysimum repandum Sisymbrium irio 2 Yes 9 Malva parviflora 2 No 0.00 0.00 0 1.59 1.59 9 0.05 0.05 9 0.45 0.45 9 Antheropeas lanosum 0.23 0.08 45 0.05 0.05 9 1.59 1.59 9 0.09 0.06 18 Oncosiphon piluliferum 0.45 0.45 9 1.00 0.60 36 0.00 0.00 0 0.45 0.45 9 0.09 0.06 18 0.00 0.00 0 1.68 1.58 27 0.05 0.05 9 0.00 0.00 0 0.00 0.00 0 1.59 1.59 9 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 1.45 0.69 45 0.00 0.00 0 0.91 0.61 18 0.50 0.45 18 0.00 0.00 0 0.00 0.00 0 Lotus strigosus 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.55 0.45 27 Bowlesia incana 0.00 0.00 0 0.00 0.00 0 0.91 0.61 18 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 0.91 0.61 18 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.45 0.45 9 Festuca octoflora Walt. Amsinckia tessellata 2 Yes Eriastrum diffusum Dichelostemma pulchellum Plantago patagonica 1, 2 Yes 1, 2 Yes Cryptantha sp. 2 Poa spp. 1, 2 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.05 0.05 9 Ambrosia deltoidea 1, 2 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.00 0.00 0 Filago spp. 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.00 0.00 0 Lupinus arizonicus 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.00 0.00 0 0.00 0.00 0 Chorispora tenella 0.00 0.00 0 0.45 0.45 9 0.00 0.00 0 0.00 0.00 0 Eriogonum wrightii 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 Uropappus lindleyi 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 0.45 0.45 9 0.05 0.05 9 0.00 0.00 0 0.23 0.08 45 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 0.05 0.05 9 0.00 0.00 0 0.00 0.00 0 0.00 0.00 0 0.05 0.05 9 0.00 0.00 0 Acacia greggii Crassula connata Yes Yes 1, 2 Yes 1 Yes Allionia incarnata L. Ambrosia confertiflora 1, 2 Yes a Yes = ethnographic or archaeological sources document prehistoric or early historic use of plant species. No = ethnographic or archaeological sources document that species was not used prehistorically. Blank = no ethnographic or archaeological information is available on that species. 1 Hodgson 2001. 2Rea 1997. 10 N = 11 quadrats per patch type. Species sorted by average cover and constancy (% of quadrats in which a species is present) across all patch types. Lowercase letters represent significant differences within rows (one-way ANOVA). 11 Appendix E. Seasonal Properties and Processes of Surface Soils across the Cave Creek Archaeological Complex Variable MANOVA Variable aMultiple Mean SE Sig. comparisons Transformation used: 4 √(x) Total inorganic N (µg N cm-2) Spring 2008 Between alignments Behind alignments Between silt fields Silt fields 21.942 25.683 12.117 20.667 1.7 2.8 0.9 2.1 <0.001 a a b a Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields 89.433 83.975 18.892 35.642 91.075 117.592 40.692 38.583 13.207 10.402 4.246 9.899 13.2 12.4 3.9 5.5 14.2 18.6 6.5 5.7 1.1 2.3 1.4 2.0 <0.001 a a b b a a b b a ab b a NO3--N <0.001 Transformation used: 3 √(x) cm-2) Extractable (µg N Spring 2008 Between alignments Behind alignments <0.001 16.1 17.9 1.0 1.3 <0.001 a a Mean Extractable NH4+-N (µg N cm-2) Spring 2008 Between alignments Behind alignments Between silt fields Silt fields Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields PO43- MANOVA Multiple Sig. comparisons SE Transformation used: 3 √(x) 5.8 7.8 5.1 4.8 1.2 1.9 0.7 1.7 0.239 a a a a 42.1 44.0 10.9 22.0 11.3 20.1 5.2 9.3 10.4 7.4 3.1 6.2 5.7 7.1 2.9 3.3 1.5 3.2 0.7 1.3 1.2 1.5 1.2 1.0 <0.001 a a b b a a b ab a ab b ab <0.001 Transformation used: 3 √(x) cm-2) Extractable (µg P Spring 2008 Between alignments Behind alignments <0.001 91.5 118.8 16.8 14.9 0.026 a a 12 Between silt fields Silt fields Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields Potential net N mineralization (µg N cm-2 d-1) Spring 2008 Between alignments Behind alignments Between silt fields Silt fields Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments 7.1 15.9 0.5 1.6 47.3 40.0 8.0 13.7 79.7 97.5 35.5 29.3 2.9 3.0 1.1 3.7 8.0 5.9 1.6 2.5 13.4 16.8 6.3 4.5 0.6 1.9 0.4 1.1 b a <0.001 <0.001 0.074 a a b b a a b b a a a a Transformation used: x 6.3 9.3 2.3 4.3 0.461966818 0.894719712 0.306861668 0.559943159 <0.001 a b c ac 5.4 2.27825303 4.7 0.876186769 0.9 0.202678812 2.3 1.0 4.1 0.615513122 0.071 a a a a a 0.043 Between silt fields Silt fields Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields Potential net nitrification (µg N cm-2 d-1) Spring 2008 Between alignments Behind alignments Between silt fields Silt fields Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments 78.5 54.7 15.9 6.6 78.1 87.8 60.4 40.0 102.4 118.4 88.2 55.7 54.1 69.0 36.1 30.5 9.8 10.4 16.0 4.9 9.6 15.6 16.3 2.9 5.5 10.3 7.7 2.5 a a 0.006 0.001 0.001 a ab ab b a a ab b ab a b b Transformation used: 3 √(x+2) 6.7 9.9 2.7 4.7 0.5 1.0 0.3 0.5 <0.001 ab a c bc 9.4 8.9 2.0 4.5 5.2 2.3 1.0 0.3 1.1 0.7 <0.001 a a b ab a 0.007 13 Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields 3.1 1.4 2.5 3.6 5.3 2.6 3.7 0.899915865 0.432983834 0.473571558 0.475245874 0.710573844 0.391876495 0.771748438 0.019 a a a a a a a Transformation used: Log(x) Herbaceous biomass (g m-2) Spring 2008 Between alignments Behind alignments Between silt fields Silt fields 101.3 132.3 89.6 103.4 6.5 16.8 12.7 7.1 0.068 a a a a Spring 2009 Between alignments Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields 52.3 63.4 41.4 55.4 142.9 146.3 83.0 75.1 4.0 6.0 2.9 3.5 11.2 19.8 12.6 10.2 0.01 ab a b ab a a b b <0.001 Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields Soil moisture (%) Spring 2008 Between alignments Behind alignments Between silt fields Silt fields Summer 2008 Between alignments Behind alignments Between silt fields Silt fields Fall 2008 Between alignments Behind alignments Between silt fields Silt fields Spring 2010 Between alignments Behind alignments Between silt fields Silt fields 5.1 1.8 3.3 4.5 5.7 2.2 4.2 0.9 0.5 0.5 0.5 0.6 0.4 0.8 0.001 ab b ab ab a b ab Transformation used: Log(x) 11.5 0.571749972 12.3 0.811321466 8.8 0.34909484 14.5 0.486706277 1.7 1.8 0.9 2.1 2.7 2.4 1.1 3.3 6.6 5.5 4.0 6.9 0.084495212 0.097572195 0.056840134 0.051239836 0.105048881 0.1443087 0.11806813 0.169423436 0.492075634 0.746802573 0.304840942 0.283478982 <0.001 a ac b c <0.001 a ac b c a a b a a ab b a <0.001 <0.001 14 Post-hoc Tukey test unless equal variance assumptions were violated (Levene's < 0.05 within a season). In these cases, the Games-Howell post-hoc test was used. For all variables, α = 0.0125 for soils, and α = 0.0167 for biomass; signficant differences between patch types within a season indicated by different lowercase letters. a N = 12 plots per feature type. 15