383-1452-1-PB
advertisement

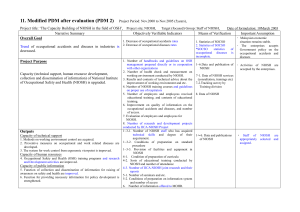
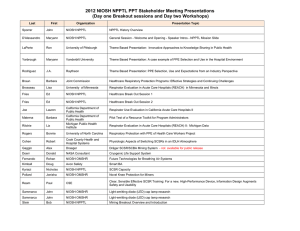
Science Administration across Divisions, Laboratories, and Offices of the National Institute for Occupational Safety and Health Maryann D’Alessandro, Ann Berry, John Decker, Amia Downes, and Amanda Harney Background HHS/CDC’s National Institute for Occupational Safety and Health (NIOSH) is the federal agency responsible for conducting research and making recommendations for the prevention of work-related injury, illness and death. NIOSH has a diverse portfolio of scientific activities including basic research, clinical/applied occupational safety and health research, systems research, policy research and applications, and several Congressionally mandated service programs. NIOSH has a professionally diverse staff of 1,200 scientists assigned between its split Office of the Director in Washington, D.C. and Atlanta, GA and six locations across the nation. NIOSH has a well formulated process to establish research priorities and conduct its Congressionally mandated service programs. Research priorities are established by considering multiple inputs to the research process. For example, NIOSH incorporates suggestions and input provided through strong stakeholder partnerships (often as steering committees) involving federal agencies, unions, labor, manufacturers, academia, and other interested parties. High level scientific reviews provide another input to NIOSH programs including over twelve National Academies' reviews conducted over the past five years and a well-established and rigorous peer review process for all research activities. NIOSH also has a Board of Scientific Counselors which provides guidance on the Institute's research activities. In an effort to maintain a central information repository to track the wide array of research and support activities in which NIOSH staff are involved, the Institute developed and continually improves the NIOSH Project Planning and Management system (NPPM). This system allows system users internal to NIOSH to analyze project and some program level data such as funding, personnel time, goals, outputs, and outcomes. Research prioritization identifies relevant research focus; however, each NIOSH Division, Laboratory, or Office (DLO) is permitted to establish and use its own processes and procedures to define its annual portfolio of specific research projects and support activities that populate the NPPM. It has been assumed that great similarity exists across the Institute as to how these annual activities are conducted. However, there has been no recent analysis of how these processes are defined. Initiative Objective The NIOSH Science Administration Health of Science (HoS) Committee defined and conducted an initiative to gather information regarding how each DLO determines its annual plans for specific research and service activities. An analysis of this information is being used to produce “best practices” that serve as a tool to identify potential approaches to enhance individual program/budget formulation processes. Further, these results will be used to develop additional HoS metrics in support of the broader goal to measure the performance and improve the capacity of the NIOSH research effort. 1 Methodology The initiative is being conducted in five steps: (1) an initial survey of presently used processes via an interview (60-90 minutes) with key representatives of each DLO, including determining the existence and status of process documentation; (2) a review of the survey results and followup activities to clarify initial information and define additional requests to ensure that all available and pertinent information has been obtained; (3) an analysis of the data; (4) a report on NIOSH program and budget formulation processes, including the definition of best practices; and (5) presentation and discussion of the conclusions with NIOSH senior management. Data Collection Initial survey interviews with the twelve NIOSH DLOs were completed between SeptemberNovember 2010. The interviews were conducted against a uniform set of questions designed to fully define the annual budget and program formulation process each organization uses: (1) Documentation of current process; (2) Schedule for making decisions; (3) Assignment of roles; (4) Evaluation criteria; (5) Process for ongoing projects; (6) Process for new start projects; (7) Participation in emergency response activities; and (8) Management of emerging issues. Followup information was obtained to clarify gaps. Preliminary Analysis and Results Even before data collection was completed it became apparent that wide variability existed across the Institute as to the degree of formality different DLOs used for their annual planning cycles. This was clearly demonstrated by the varied documentation for the majority of the processes used. This can be explained in part because of the different funding sources that are available to each respective group. Thus, the potential to receive funds from NIOSH’s intramural National Occupational Research Agenda (NORA) competition process (a primary source of funds for many organizations) was premised upon complying with a well-documented and scheduled process managed by the NIOSH NORA Coordination Office. However, project selection in support of other programmatic initiatives (e.g., requiring the discretionary funds) were subject to varying levels of rigor and comparative analysis. More significantly, few of the surveyed DLOs conducted a unified process where all of its research and support activities were “competitively” analyzed for the purpose of formulating its future year’s program and support activities. A similar diversity was found in the specific attributes of the various processes used by each organization, i.e., evaluation criteria, management of emerging issues. Almost every unit conducted a rigorous mid-year review of the status of on-going projects. These reviews uniformly involved broad participation of researchers, project leaders, supervisors, branch chiefs, and the organization’s senior management. Varying degrees of formality of documentation were employed, but each review was conducted in an exacting manner. It can be inferred that these reviews did provide information useful to subsequent funding decisions. However, few made a direct and formal connection between the two. Many organizations used written quarterly reports as an additional way to monitor performance. All were paper-based reviews. 2 Across the Institute there was uniformity in complying with the requirements for project reporting in NIOSH’s NPPM system. Compliance with its timing requirements often served as the planning schedule for the various units. Best Practices A number of Best Practices have been identified. The best practices will made available for consideration and adoption into each organization’s management system. 3