The principle of DGT - Springer Static Content Server
advertisement

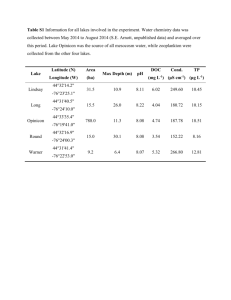
1 Electronic Supplementary Material 2 3 Sampling of ammonium ion in water samples by using the diffusive gradients in thin 4 films technique (DGT) and a zeolite based binding phase 5 Zhongmin Feng, Tingting Guo, Zhiwen Jiang, Ting Sun* 6 College of Sciences, Northeastern University, Shenyang 110819, China 7 *Corresponding author, E-mail: sun1th@163.com, Tel.: +86 24 83684786 8 9 10 11 The principle of DGT The analyte concentration sampled by DGT is based on the Fick's first law of diffusion. Mass flux (J) of an ion passing through the diffusive layer to the binding gel is expressed as: 12 13 Where D is diffusive coefficient of analyte through diffusive gel, ∂C/∂x is the concentration 14 gradient, Δg is the thickness of diffusive layer and filter, δ is the thickness of the diffusive 15 boundary layer, C is the analyte concentration in the waters and C' is the analyte concentration 16 at the boundary between the diffusive layer and binding layer. When the DGT devices are 17 deployed in well stirred waters, the diffusive boundary layer δ can be neglected compared to 18 the thickness of the diffusive layer. Thus, Eq. S1 becomes Eq.S2. 19 20 Flux (J) can also be described as the mass (M) of analyte passing through diffusive layer of an 21 area (A) in a given time (t). 22 23 Combining Eq.S2 and S3, the Eq.S4 is obtained as follows: 24 25 The above equation states an important feature of DGT is its ability to determine the analyte 26 concentration CDGT through the measured mass immobilized on binding gel and the 1 1 deployment time. The analyte is eluted from binding gel with suitable solution and M is 2 calculated by Eq. (S5). 3 4 Where Ce (mg L-1) is the concentration of analyte in the eluted solution, Ve and Vgel (mL) are 5 the volume of the elute solution and binding gel respectively, and the fe is elution efficiency 6 from the elution. For unknown the exact diffusion coefficient for ammonium (NH4+-N) in 7 diffusive layer of DGT devices (hydrogel), measurement of diffusion coefficient of NH4+-N is 8 carried out in this study under environmental temperature and pressure study. 9 10 Experimental procedures 11 Preparation of diffusive gel and binding gel 12 In order to minimize the reactions between diffusive gels and target analyte (NH4+-N), 13 diffusive gel solutions were prepared with 15 % acrylamide (UP grade, Amresco) (w/v) and 14 0.75 % N,N'-methylenebisacrylamide (HP grade, Amresco) (w/v). After well stirred, 20 μL 15 10 % (w/v) NaHSO3 catalyst and 150 μL potassium persulphate (5%, w/v) initiator were 16 added into 8 mL diffusive gel solution. And then, the solution was immediately cast between 17 two glass plates and horizontally placed in an oven at 50 °C to polymerize for 1 h. The 18 diffusive gels were carefully taken away from glass plates, soaked in deionized water for 24 h 19 to remove unpolymerized monomer and stored in 0.01 M NaNO3 for use. After hydrolyzation, 20 the thickness of the diffusive gel was measured accurately by electronic digital display 21 micrometer (211-101F, Guilin Guanglu Measuring Instrument Co., Ltd., China) and remained 22 at 1.0 mm. 23 Micro-sized zeolite 13X powder (Beijing Huaye huanyu Chemical Co.,Ltd., China) was 24 used for binding gels. First, 2 g zeolite powder was dissolved in 8 mL gel solution composed 25 of 28.5 % acrylamide (w/v) and N,N'-methylenebisacrylamide (w/v). Then, followed by the 26 addition of catalyst and initiator as described above, the resulting mixture was cast between 27 two glass plates and set at 50 °C for 1 h. The Na- zeolite binding gels were prepared by 2 1 soaking binding gels into 0.5 M NaCl for 24 h. After that, the gels were rinsed several times 2 and stored in deionized water for use. After hydrolyzation, the thickness of the gel was 3 measured accurately by electronic digital display micrometer and remained at 0.5 mm. 4 Measurement of diffusion coefficient 5 The diffusion coefficient of ammonium in the diffusive gel was measured by diffusion 6 cell as stated previously. The diffusion cell was made up of two compartments (X and Y) 7 which connected by a 1.8 cm diameter window filled with a diffusive gel (1.0 mm). The 8 compartment X was used a solution of 100 mg N L-1 (NH4Cl, pH 6.0) and the compartment Y 9 was used deionized water. Each side of the diffusion cell was well stirred with an electric stir 10 bar. 1 mL solutions were removed from both compartments at intervals of 15-20 min. 11 Meanwhile, the corresponding volume of the original source solution and deionized water 12 were supplemented. The mass of nitrogen diffused from compartment X to Y was measured 13 directly in compartment Y and increased linearly with time. The salt NH4Cl diffusion 14 coefficient Ds was calculated from a slope of mass of N diffused through the diffusive layer 15 according to time, values of C and A. 16 17 Where C is the initial concentration of NH4Cl solution in compartment X, A is the window 18 area. Suppose that the diffusion coefficient of potassium ion in the diffusive gel is the same as 19 in the aqueous solution, the ammonium diffusion coefficient, D, is revised by Eq. (S6). 20 21 To evaluate the effect of pH on the diffusion coefficient, a batch of diffusion cell tests 22 were done at pH range of 3~10 with ionic strength of 0.01 M at room temperature. To 23 investigate the ionic strength on the diffusion coefficient, a batch of diffusion cell tests were 24 operated in the ionic strength range of 0.01 ~ 100 mM NaNO3 at pH 6 (room temperature). 25 Selective adsorption by zeolite binding gel 26 Competitive binding of ions to the binding gels was examined by placing the discs in 20 27 mL mixture solution of 10 mg L-1 NH4+, Na+, K+, Mg2+, Ca2+, Ba2+ and Al3+ at room 3 1 temperature for 12 h. The initial concentrations and finial concentrations of NH4+-N were 2 measured using Nessler's Reagent method, and other ions were detected by an ICP-AES 3 method. Binding selectivity of the gels was calculated according to the ratio between the 4 amount of ammonium adsorption on the gel ( ) to that of other metal ions (Mm). 5 6 Interference of metal ions 7 To evaluate the possible effect of various metal ions (K+, Mg2+,Ca2+ and Al3+) that were 8 widespread in surface waters on the zeolite-DGT uptake of NH4+-N, the DGT devices were 9 deployed for 12 h and 24 h at pH 6.0 (0.001 M NaNO3) in 2 L well stirred solution that 10 contained 1 mg N L-1 (NH4Cl) (this N concentration is typically found in many surface waters 11 [1-3]) and respectively mixed with rising concentrations of K+ (as KCl; 4, 20, 80, 120 mg L-1), 12 Ca2+(as CaCl2; 20, 40, 80, 120 mg L-1), Mg2+ (as MgCl2; 15 ,60, 120, 240 mg L-1), and Al3+(as 13 AlCl3; 15, 60, 120, 240 mg L-1) under room temperature. 14 15 Interference of anions 16 To evaluate the possible effect of NO3- and NO2- that were widespread in surface waters 17 on the zeolite-DGT uptake of NH4+-N, the DGT devices were deployed for 24 h at pH 6.0 18 (0.001 M NaNO3) in 2 L well stirred solution that contained 1 mg N L-1 (NH4Cl) and 19 respectively mixed with rising concentrations of NO3- (as Mg(NO3)2; 15, 60, 120, 240 mg L-1) 20 and NO2- (as NaNO2; 30, 60, 80,120 mg L-1) under room temperature. 21 Results and discussion 22 Influence of pH and ionic strength on diffusion coefficient 23 For a constant ionic strength, the diffusion coefficient of NH4+-N was not appreciably 24 affected by the pH of aqueous solution at the range of 3~10 (Fig. S1). It is well known that, 25 according to Eq. (1), the proportion of NH3.H2O increased with the pH of waters. It is 4 1 hypothesized that both the NH4+ and NH3.H2O can diffuse through the filter and diffusive gels. 2 For a constant pH, the diffusion coefficient of NH4+-N was dependent on low ionic strength of 3 aqueous solution (0.01 to 1 mM NaNO3) (Fig. S2). The above results suggest no measurable 4 charge effect or specific binding in the diffusion process. Note that a slight decreased 5 diffusion coefficient of NH4+-N was obtained with increasing concentrations of NaNO3 (from 6 0.01 to 0.1 M), which may be caused by the viscosity effect[4].In summary, accurate 7 determination of ammonium concentration by DGT would be achieved, at least for the range 8 of conditions. 9 Selectivity 10 It is necessary to evaluate the competing binding of alkali metals and alkaline earth metal 11 ions to the zeolite gels before it was utilized for practical purpose. Fig.S3 shows the 12 selectivity adsorption properties for NH4+-N over other ions. The adsorption selectivity order 13 was NH4+ > K+ > Ba2+ ≈ Ca2+ > Al3+ > Mg2+ when all the ions were present in the solution at 14 the same concentration. The adsorption result by zeolite gel for Na+ was not given because it 15 was difficult to exactly determine the Na+ concentration difference resulting from sodium 16 form of zeolite (Na-zeolite). Note that the selectivity order was in relation to ionic radius. 17 That is might because the available pore size of zeolite 13X is suitable for NH4+-N as well as 18 cations with the similar radius. 19 Effects of anions 20 NO3- and NO2- commonly present in natural waters can compete with ammonium for 21 sorption sites in zeolite gel and affect the N sampling using the zeolite-DGT technique. Hence, 22 the influences of NO3- and NO2-on the DGT sampling of N were evaluated by zeolite gels for 23 24 h. Over the NO3- (15,60, 120 mg L-1) and NO2- (30, 60, 80 mg L-1) concentration range 24 investigated, there was good agreement between the predicted concentration (CDGT) calculated 25 using Eq. (S4) and the concentration of NH4+-N (CSol) directly measured in bulk solution by 26 Nessler's Reagent method, with a ratios of CDGT/CSol in the range of 0.90~1.10 (Fig. S6). The 27 experimental results indicated that zeolite-DGT performed well within these concentrations 5 1 range of anions. By comparison, the tolerant concentration limit of NO3- in the current study 2 was approximately 10 times as high as those of lake water [5], and for NO2- the upper 3 concentration limit was about 100 times higher [6]. 4 5 Table S1. The properties of zeolite 13X Zeolite 13X Structural formula Na2O.Al2O3.(2.8±0.2) SiO2.(6~7) H2O SiO2 /Al2O3 2.6~3.0 Effective aperture (Å) 10.0 Effective diameter (μm) 1.0 Manufacture Beijing Huaye huanyu Chemical (http://www.bjchemmart.com.cn/) 6 7 Table S2. ICP-AES operating parameters Instrument Optima4300 DV Output power 1.1 kw Auxiliary gas flow rate 0.8 L min-1 Cooling gas flow 18.0 L min-1 Nebulizer 1.0 L min-1 Replicates of each analysis run 3 Sample uptake delay 30 s 8 9 6 Co.,Ltd., China 1 Table S3. Composition of synthetic freshwater synthetic freshwater a Compositon pH 6.9 Na+ (mg L-1) 33.16 K+ (mg L-1) 3.80 Mg2+ (mg L-1) 16.10 Ca2+ (mg L-1) 76.20 Cl- (mg L-1) 48.00 SO42- (mg L-1) 110.00 NH4+-N(mg L-1) 2 a 1.0 Prepared according to Dong et al.[7]. 3 4 Table S4. Compositions of three natural waters for DGT application Compositon 5 Wanquan fishpond water Hunhe river water Nahu lake water pH 6.7 7.1 6.9 DOC (mg C L-1) -a 12.1 8.8 Na+ (mg L-1) 38.00 19.50 33.16 K+ (mg L-1) 6.86 1.50 3.80 Mg2+ (mg L-1) 14.04 12.30 16.10 Ca2+ (mg L-1) 38.05 51.00 76.20 Cl- (mg L-1) 40.02 29.00 48.00 SO42- (mg L-1) 120.00 200.00 110.00 TP (mg P L-1) 1.00 0.20 0.25 NO3--N (mg N L-1) 7.51 1.65 6.59 NO2--N (mg N L-1) 0.40 0.15 0.25 NH4+-N(mg L-1) 1.34 0.63 0.29 a not detected 6 7 1 2 Fig.S1. Effect of pH on diffusion coefficient. Data was obtained at 18 °C and 0.001 M NaNO3. 3 Error bars represent the standard deviation of repeated experiments and measured to be 4 between 5 %~11 % (n=3). 5 6 Fig.S2. Effect of ionic strength on diffusion coefficient. Data was obtained at 18 °C and pH 6. 7 Error bars represent the standard deviation of repeated experiments and measured to be 8 between 5 %~10 % (n=3). 9 8 1 2 Fig.S3. Selectivity of the zeolite binding gels for ammonium over other cations as a function 3 of their ionic radius (pH=6.0). Binding selectivity of the gels was calculated according to the 4 ratio between the amount of ammonium adsorption on the gel ( 5 ions (Mm). ) to that of other metal 6 7 Fig.S4. Elution efficiency of NH4+-N removing from zeolite gels using NaCl solution of 8 different concentrations. The error limits are standard deviation calculated using 6 replicates. 9 9 1 2 Fig.S5. DGT response under various cationic interferences. CDGT and CSol are the 3 concentration of ammonium ion by zeolite-DGT technique and Nessler's Reagent method, 4 respectively. 5 6 7 8 Fig.S6. DGT response under various anionic interferences. CDGT and CSol are the 9 concentration of ammonium ion by zeolite-DGT technique and Nessler's Reagent method, 10 respectively. 10 1 Reference 2 1. Zhou L, Boyd CE (2015) An assessment of total ammonia nitrogen concentration in 3 Alabama (USA) ictalurid catfish ponds and the possible risk of ammonia toxicity. Aquaculture 4 437:263-269. doi:http://dx.doi.org/10.1016/j.aquaculture.2014.12.001 5 2. Guo L, Chen Q, Fang F, Hu Z, Wu J, Miao A, Xiao L, Chen X, Yang L (2013) Application 6 potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal 7 of 8 doi:10.1016/j.biortech.2013.05.021 9 3. Xia XH, Yang ZF, Huang GH, Zhang XQ, Yu H, Rong X (2004) Nitrification in natural 10 waters with high suspended-solid content - A study for the Yellow River. Chemosphere 57 11 (8):1017-1029. doi:10.1016/j.chemosphere.2004.08.027 12 4. Y.-H. Li, S. Gregory (1974) Diffusion of ions in seawater and in deepsea sediments. 13 Geochim Cosmochim Acta 38:708-714 14 5. Sappa G, Ergul S, Ferranti F, Sweya LN, Luciani G (2015) Effects of seasonal change and 15 seawater intrusion on water quality for drinking and irrigation purposes, in coastal aquifers of 16 Dar 17 doi:http://dx.doi.org/10.1016/j.jafrearsci.2015.02.007 18 6. Hong H, Qian L, Xiong Y, Xiao Z, Lin H, Yu H (2015) Use of multiple regression models 19 to evaluate the formation of halonitromethane via chlorination/chloramination of water from 20 Tai 21 doi:http://dx.doi.org/10.1016/j.chemosphere.2014.06.084 22 7. Dong J, Fan H-T, Sui D-P, Li L-C, Sun T (2014) Sampling 4-chlorophenol in water by 23 DGT technique with molecularly imprinted polymer as binding agent and nylon membrane as 24 diffusive layer. Anal Chim Acta 822:69-77. doi:10.1016/j.aca.2014.03.015 eutrophic es Lake lake Salaam, and the water. Tanzania. Qiantang Bioresource J Afr River, China. 25 26 11 Earth Technology Sci Chemosphere 105 119 142:45-51. (0):64-84. (0):540-546.