Amendment to Change in Personnel
advertisement

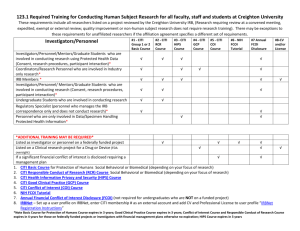
Amendment: Change in Personnel (CIP) [Version 1/22/2016] Request to remove or add personnel to IRB, IACUC, and IBC Projects INSTRUCTIONS: ALWAYS download the latest version. DO NOT make any changes to this form. Complete appropriate sections and submit this form in its original format to one of the following addresses. IRB projects, submit this form AND all required supporting documents to IRBoffice@lsuhsc.edu or by campus mail #RCB206. IACUC projects, email this form to IACUCoffice@lsuhsc.edu Projects not associated with an IRB or IACUC, email this form to IBCoffice@lsuhsc.edu Electronic Submission Date: Principal Investigator: Email: Office Telephone: Copy email correspondence to: at Email: [Coordinator/Co-investigator submitting forms/documents in behalf of the PI must cc the PI on the email.] By submission of this amendment, the Principal Investigator of this project attest that the information below is accurate and complete and attests to the following statements. I accept the responsibility for the safe conduct of work with this study at the Biological Safety Level practices and procedures assigned by the IBC. I will inform all personnel, who may be at risk of potential exposure of the conditions of this work. I assure that all personnel will receive adequate training to perform all activities safely and proficiently. I will not carry out the work described in the attached application until it has been approved by the IBC/IACUC/IRB, where applicable, and all requirements have been met. Where applicable, I agree to comply with the NIH requirements pertaining to conducting research and the shipment and transfer of recombinant DNA materials. I acknowledge my responsibility for the conduct of this research in accordance with LSUHSC-NO policies and the NIH Guidelines. This amendment was administratively approved. Annual re-approval will be bound to the approved date of the application which this document amends. __________________________________________________ Approval Signature ______________________ Date of Approval 1. Complete the table for all applicable projects affected by this change. IBC # A# IACUC# A# IRB# A# Project Title 2. To remove individuals from participation, list their full name. If removing investigator(s) from an IRB study whose name was previously listed on the consent form, attach a revised consent form. If only removing personnel at this time, STOP here and submit the form(s). 3. To authorize participation of an individual, provide the demographic information of the individual and complete applicable items 4 through 7. Name: Degree: School of: Department of: Email: Office Phone: Mobile Phone: Campus Office Address and Room Number: Fax: If Off-Campus, provide US Mail Address: Indicate personnel status/classification (check all that applies): Faculty Staff Gratis Appointment Non-LSUHSC-NO (complete #4) Medical Student Graduate Student Fellow Resident For student/fellow/residents, indicate the type of project. Project is a thesis or Capstone/Dissertation (Masters/PhD) Project is for the course: Other – Explain: 4. Approval by the LSUHSC-NO IRB for LSUHSC-NO employees, appointees, and students does not extend to individuals on the project who are not LSUHSC-NO employees, appointees, or students. Non-LSUHSC individuals must seek IRB review from their IRB of record. If the individual you wish to add to the study does not have an IRB of record, contact the LSUHSC-NO IRB before submitting this form. For those individuals that have an IRB of record, provide the following information. a) b) c) d) e) Institutional Affiliation: Department: Institution’s Mailing Address: IRB of Record: (Submit a copy of the approval when available.) Submit a copy of the individual’s CV. Submit documentation of required training (see table in #7). 5. Indicate the role and list the activities the individual is trained and authorized to perform in this project. a) Role: Co-investigator Study Coordinator/Clinical Student Investigator Study Coordinator/Data b) Activities this individual has been trained to perform: Lab Technician/Assistant Other, specify: 6. If amending an IRB protocol, indicate what other required IRB documents are included with this form. (The IRB full Demographic Form is not required when using this form). Conflict of Interest Attestation Form (Required for all team members) Attestation Signature Page from IRB Demographic Form (Required when adding Co-investigators) Certification of Department Review (Required when changing PI) Revised informed consent form(s) (Required when changing PI and/or Co-investigators) NOTE: IRB CIPs will not be processed unless the above required documents are submitted as one packet in an email to IRBoffice@lsuhsc.edu or by paper submission to the IRB Office, 433 Bolivar St. #206, NOLA 70112 7. In the following training table, enter the date of completion of training required to participate in research and to perform the activities listed above. Enter any other relevant training received. To determine which training modules are required, refer to the training guide on the ORS webpage: http://www.lsuhsc.edu/administration/academic/ors/docs/TableTrainingRequiredResearch.pdf Contact your Department Manager or email IBCoffice@lsuhsc.edu for online KDS training completion dates. Individuals can supply their CITI, Animal Care and other specific training dates. Individuals without KDS access and non-LSUHSC participants may submit documentation of comparable training from other institutions or may download the self-study courses from ORS web page: http://www.lsuhsc.edu/administration/academic/ors/training.aspx Name of Personnel: List PI’s Lab-Specific, SOPs, or Other Training Received: From PI or Lab Manager Date Completed Enter who and when personnel was oriented to the protocol, lab, and/or clinic Other specific training: Other specific training: EH&S and Biological Training Module Bloodborne Pathogen – (High Risk required for researchers; Low Risk for all others) Biological Safety Basic Training (Required when working with humans, animals or Type KDS HR annual or LR every 5 years biological materials, toxins, pathogens, radiation, rDNA) KDS every 3 years IBC and rDNA Compliance Program (Required for PI & key personnel) KDS once Shipping Biological Materials (Personnel preparing shipments) EH&S once Radiation Safety (Required where applicable) EH&S once Laser Safety (Required for Class 3B or 4 lasers) EH&S once Laboratory Safety (General/Basic Training) (Required if working in a lab) KDS once Date Completed Other training: Institutional Required Training Modules KDS Date Completed Conflict of Interest in Sponsored Projects (Required for all team members participating in research projects, whether sponsored or not, human, animal or other) KDS every 4 years HIPAA Privacy in Research (human research projects) KDS every 3 years Human Subject Research Training CITI Date Completed Complete applicable courses related to type of research to be performed, initial and then the Refresher every 3 years Biomedical Research - Initial Course Choose at least one elective: Social and Behavioral Research for Biomedical Researchers Vulnerable Subjects - Involving Prisoners Vulnerable Subjects - Involving Children Vulnerable Subjects - Involving Pregnant Women, Human Fetuses, Neonates Internet Research - SBR Biomedical Research - Refresher Course Social & Behavioral Research - Initial Course Choose at least one elective: Research with Prisoners - SBR Research with Children - SBR Research in Public Elementary and Secondary Schools - SBR Vulnerable Subjects - Involving Pregnant Women, Human Fetuses, Neonates Internet Research - SBR CITI once – For: staff involved in: clinical trials drug/device trials tissue use or banking medical chart review any medically-oriented investigation CITI every 3 years CITI once – For: staff involved in: survey/questionnaire focus groups interview psychological or other testing educational testing epidemiological reviews Social & Behavioral Research-Refresher Course GCP - Drug Development Gradebook - Initial Course: Conducting Studies According to FDA Regulations & Good Clinical Practices CITI every 3 years CITI once GCP - Drug Development Gradebook - Refresher Course GCP - Device Development Gradebook - Initial Course: Conducting Studies According to FDA Regulations & Good Clinical Practices CITI every 3 years CITI once GCP - Device Development Gradebook - Refresher Course Other specific training: Other specific training: CITI every 3 years Animal Research Training DoAC or CITI Division of Animal Care (DoAC) Occupational & Health Risk Assessment for Exposure to Animal Use or Care (Anyone exposed to research animals) DAC Form - Initial DAC Orientation (Required for access into any part of the Animal Care facilities) required, update as needed Classroom DAC Aseptic Surgery (Required if performing survival surgery) Classroom Other training assigned by veterinarian: Working with the IACUC (Investigators, Staff, Students) -Initial Course CITI once Working with the IACUC (Investigators, Staff, Students) -Initial Course CITI once Working with Animals In Biomedical Research-Refresher Course CITI every 3 years Complete applicable courses related to activities to be performed. (Required only once) Aseptic Surgery CITI Reducing Pain and Distress in Laboratory Mice and Rats CITI List Species Working with in Research Settings: List Species Working with in Research Settings: CITI List Species Working with in Research Settings: CITI Other specific training: Other specific training: CITI Date Completed