Solutions to Problems Give the number of significant figures in each

Solutions to Problems
Give the number of significant figures in each of the following:
2
7
3
3
4
2
2
3
1
3
5
2
Multiply each of the following, observing significant figure rules:
5500 m 2 7300 m 2
40 in 2
.038 in 2
5.1 m 2
3890000cm 2
Divide each of the following, observing significant figure rules:
47 m/s 3.8 mi/hr
.437 g/mol 99 m/s
Add each of the following, observing significant figure rules:
3.0 m 106.89 g 111 cm
Subtract each of the following, observing significant figure rules:
41.09m 10.86 g 172.1 cm
Work each of the following problems, observing significant figure rules:
Average percent = 7.36%
V = 320 cm 3 m = 46593.6 g = 46600 g m = 177000 g
Significant Figures Practice
Part I:
1.
5
2.
2
3.
5
4.
3
5.
5
Part II:
6.
7.85 x 10 -3
7.
1.007 x 10 3
8.
1.53101 x 10 4
9.
3.5006700 x 10 7
10.
3.1700 x 10 -2
Part III:
11.
.00137
12.
2907000
13.
7500
14.
.00004301
15.
900.0
Part IV:
16.
139.3 mm
17.
217.2 g
18.
112.2 kg
19.
-129.04 m
Part V:
20.
173.2 mm 2
21.
.91 m 2
22.
13.3 m/s
23.
15 dollars/lb
Percent Error Practice Sheet
1.
16.6% = 17%
2.
8/40% = 8%
3.
11.1% = 10%
4.
10.0% = 10%
5.
15.4% = 15%
6.
13.2%
7.
16.5%
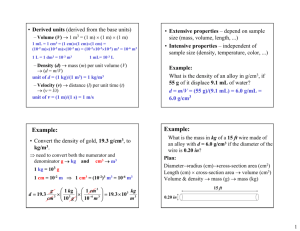
Density Practice Problems
1.
D = 2.70 g/mL
2.
D = 13.6 g/mL
3.
m = 158 g
4.
D = 8.9 g/cm 3
5.
D = 1.59 g/mL
6.
D = 1.84 g/mL
7.
D = 220 g
8.
D = 11.3 g/cm 3
9.
D = 7.92 g/mL
10.
V = 238 cm 3
Practice Worksheet
1.
State the number of significant digits in each measurement a.
3 b.
4 c.
4 d.
3
2.
Round the following numbers as indicated:
To four figures: a.
3.682 b.
21.86 c.
112.5
To one decimal place: a.
2 b.
6 c.
8
To two decimal places: a.
0.031 b.
3.4 c.
42
3.
Solve the following problems and report answers with appropriate number of significant digits: a.
26.3 cm b.
1614 m c.
0.5 g d.
Cannot do e.
3.0 cm 2 f.
2.73 cm/s g.
3.07708 g
4.
Express in scientific notation: a.
1.238763 x 10 5 b.
1.236840 x 10 6 c.
2.11 x10 -13 d.
2.38 x 10 -4
5.
Identify the sums or differences of the following: a.
1.81 x 10 5 b.
9.1 x 10 1 c.
1.2 x 10 4 d.
4.2 x 10 -2
6.
Express the product and the quotients of the following: a.
1.50 x 10 12 b.
1.6 x 10 -1 c.
1.25 x 10 4 d.
8.2 x 10 3
7.
Fill in the blanks: a.
1,000,000 µg = 1 g b.
1,000,000 s = 1 Ms
8.
Do the following conversions. Don’t forget significant figures! a.
0.000347 g b.
6.724 x 10 1 mm c.
346800 g d.
14000 mL
Dimensional Analysis
5.
44640 min = 45000 min
6.
672 test tubes = 700 test tubes
7.
$19.74
8.
4.500 x 10 6 km/min
9.
m = 21975 mg = 22000 mg
10.
2.6785 x 10 12 µs
11.
How many: a.
86400 s b.
750000 mg c.
.00075 g d.
250000000 cm
12.
$70.31