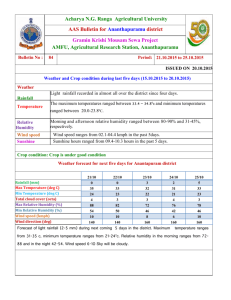
DOCX format - 389 KB - Office of the Gene Technology Regulator
advertisement

Risk Assessment and Risk Management Plan for DIR 131 Limited and controlled release of safflower genetically modified for high oleic acid composition Applicant: Commonwealth Scientific and Industrial Research Organisation February 2015 PAGE INTENTIONALLY LEFT BLANK DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Summary of the Risk Assessment and Risk Management Plan for Licence Application No. DIR 131 Introduction The Gene Technology Regulator (the Regulator) has decided to issue a licence for this application for a limited and controlled release of a genetically modified organism (GMO) into the environment. A Risk Assessment and Risk Management Plan (RARMP) for this application was prepared by the Regulator in accordance with the requirements of the Gene Technology Act 2000 (the Act) and corresponding state and territory legislation, and finalised following consultation with a wide range of experts, agencies and authorities, and the public. The RARMP concludes that this field trial poses negligible risks to human health and safety and the environment and that any risks posed by the dealings can be managed by imposing conditions on the release. The application Application number DIR 131 Applicant CSIRO Project title Limited and controlled release of safflower genetically modified for high oleic acid composition Parent organism Safflower (Carthamus tinctorius L.) Three gene fragments involved in altered fatty acid composition fragment of FATB (palmitoyl-ACP thioesterase) gene from safflower fragment of FAD2 ( Δ12 desaturase) gene from safflower fragment of another fatty acid biosynthesis gene1 from safflower Two selectable marker genes from bacteria hph (hygromycin phosphotransferase, truncated) from Escherichia coli (antibiotic resistance selectable marker) gfp (green fluorescent protein) from Aequorea victoria (visual marker) Introduced genes and modified traits Proposed locations 602 sites in Queensland, Victoria, Australian Capital Territory, New South Wales and Western Australia Proposed release size Total planting area of up to 850 hectares over 4 growing seasons: in 2015 – up to 10 sites of 5 ha each in 2016 & 2017 – up to 15 sites of 10 ha each per year in 2018 – up to 20 sites of 25 ha each Proposed release dates January 2015 – August 2019 1 The identity of this gene has been declared as Confidential Commercial Information (CCI) under section 185 of the Act. 2 Total number of sites clarified during consultation. Summary I DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Primary purpose Office of the Gene Technology Regulator To evaluate the oil content and agronomic performance of GM safflower under field conditions in various regions around Australia. To provide enough extracted oil for product development and testing in industrial processes. Risk assessment The risk assessment concludes that there are negligible risks to the health and safety of people, or the environment, from the proposed release. The risk assessment process considers how the genetic modification and activities conducted with the GMOs might lead to harm to people or the environment. Risks are characterised in relation to both the seriousness and likelihood of harm, taking into account information in the application (including proposed limits and controls), relevant previous approvals, current scientific/technical knowledge and advice received from a wide range of experts, agencies and authorities consulted on the RARMP. Both the short and long term impact are considered. Credible pathways to potential harm that were considered included: unintended exposure to the GM plant material; unintended effects of the genetic modification; increased spread and persistence of the GM safflower relative to unmodified plants; and transfer of the introduced genetic material to non-GM safflower or other sexually compatible plants. Potential harms associated with these pathways included toxicity to people and other animals, allergic reactions in people, and environmental harms associated with weediness. The principal reasons for the conclusion of negligible risks are that the proposed limits and controls effectively contain the GMOs and their genetic material and minimise exposure; the introduced genetic modifications are unlikely to cause harm to people or the environment; and genes similar to most of the introduced genes are common in the environment. Risk management plan The risk management plan describes measures to protect the health and safety of people and to protect the environment by controlling or mitigating risk. The risk management plan is given effect through licence conditions. As the level of risk is considered negligible, specific risk treatment is not required. However, as this is a limited and controlled release, the licence includes limits on the size, locations and duration of the release, as well as controls including containment provisions at the trial site; prohibiting the use of GM plant materials in human food or animal feed; destroying GM plant materials not required for further studies; transporting GM plant materials in accordance with the Regulator’s guidelines; and conducting post-harvest monitoring at the trial site to ensure all GMOs are destroyed. Summary II DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Table of Contents SUMMARY OF THE RISK ASSESSMENT AND RISK MANAGEMENT PLAN ........................................... I INTRODUCTION ...................................................................................................................................................... I THE APPLICATION .................................................................................................................................................. I RISK ASSESSMENT .............................................................................................................................................. II RISK MANAGEMENT PLAN ................................................................................................................................... II TABLE OF CONTENTS ....................................................................................................................................... III ABBREVIATIONS ................................................................................................................................................. IV CHAPTER 1 RISK ASSESSMENT CONTEXT ................................................................................................. 1 SECTION 1 SECTION 2 SECTION 3 3.1 3.2 SECTION 4 SECTION 5 5.1 5.2 5.3 5.4 SECTION 6 6.1 6.2 6.3 6.4 SECTION 7 7.1 7.2 BACKGROUND................................................................................................................................ 1 REGULATORY FRAMEWORK .......................................................................................................... 1 THE PROPOSED DEALINGS ............................................................................................................ 2 The proposed limits of the dealings (size, locations, duration and people) .............. 2 The proposed controls to restrict the spread and persistence of the GMOs and their genetic material in the environment ........................................................................... 4 THE PARENT ORGANISM ................................................................................................................ 5 THE GMOS, NATURE AND EFFECT OF THE GENETIC MODIFICATION ............................................ 6 Introduction to the GMOs ........................................................................................................ 6 The introduced genes, encoded proteins and their associated effects ...................... 7 Toxicity/allergenicity associated with the introduced safflower genes ...................... 8 Characterisation of the GMOs................................................................................................ 8 THE RECEIVING ENVIRONMENT ...................................................................................................... 8 Relevant abiotic factors ........................................................................................................... 9 Relevant agricultural practices .............................................................................................. 9 Presence of related plants in the receiving environment ............................................... 9 Presence of similar genes and encoded proteins in the environment ...................... 10 RELEVANT AUSTRALIAN AND INTERNATIONAL APPROVALS ...................................................... 10 Australian approvals .............................................................................................................. 10 International approvals .......................................................................................................... 10 CHAPTER 2 RISK ASSESSMENT ................................................................................................................... 11 SECTION 1 SECTION 2 2.1 2.2 2.3 2.4 SECTION 3 SECTION 4 INTRODUCTION ............................................................................................................................. 11 RISK IDENTIFICATION ................................................................................................................... 12 Risk source ............................................................................................................................... 12 Causal pathway........................................................................................................................ 13 Potential harm .......................................................................................................................... 14 Postulated risk scenarios...................................................................................................... 14 UNCERTAINTY .............................................................................................................................. 23 RISK EVALUATION ....................................................................................................................... 24 CHAPTER 3 RISK MANAGEMENT PLAN ..................................................................................................... 26 SECTION 1 SECTION 2 SECTION 3 3.1 3.2 SECTION 4 SECTION 5 BACKGROUND.............................................................................................................................. 26 RISK TREATMENT MEASURES FOR IDENTIFIED RISKS ................................................................. 26 GENERAL RISK MANAGEMENT ..................................................................................................... 26 Licence conditions to limit and control the release ....................................................... 26 Other risk management considerations ............................................................................ 31 ISSUES TO BE ADDRESSED FOR FUTURE RELEASES ................................................................... 32 CONCLUSIONS OF THE RARMP ................................................................................................. 32 REFERENCES ...................................................................................................................................................... 33 APPENDIX A SUMMARY OF SUBMISSIONS FROM PRESCRIBED EXPERTS, AGENCIES AND AUTHORITIES .............................................................................................................................. 38 APPENDIX B SUMMARY OF SUBMISSIONS FROM THE PUBLIC ........................................................... 41 Table of Contents III DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Abbreviations APVMA ARA CaMV CCI cm CSIRO DIR DNA FAD2 FATB FSANZ gfp GM GMO GRDC ha HGT hph LA LCPUFA m miRNA mRNA NSW OECD OGTR OA RARMP Regulations Regulator RNA RNAi siRNA the Act Abbreviations Australian Pesticides and Veterinary Medicines Authority Arachidonic acid Cauliflower mosaic virus Confidential Commercial Information as declared under section 185 of the Gene Technology Act 2000 Centimetres Commonwealth Scientific and Industrial Research Organisation Dealings involving Intentional Release Deoxyribonucleic acid Δ12 desaturase gene Palmitoyl ACP thioesterase gene Food Standards Australia New Zealand Green fluorescent protein gene Genetically modified Genetically modified organism Grains Research and Development Corporation Hectare Horizontal gene transfer Hygromycin phosphotransferase gene linoleic acid Long chain polyunsaturated fatty acids Metres microRNA Messenger RNA New South Wales Organisation for Economic Co-operation and Development Office of the Gene Technology Regulator Oleic acid Risk Assessment and Risk Management Plan Gene Technology Regulations 2001 Gene Technology Regulator Ribonucleic acid RNA interference Small interfering RNA The Gene Technology Act 2000 IV DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Chapter 1 Office of the Gene Technology Regulator Risk assessment context Section 1 Background An application has been made under the Gene Technology Act 2000 (the Act) for Dealings involving the Intentional Release (DIR) of genetically modified organisms (GMOs) into the Australian environment. The Act in conjunction with the Gene Technology Regulations 2001 (the Regulations), an inter-governmental agreement and corresponding legislation that is being enacted in each State and Territory, comprise Australia’s national regulatory system for gene technology. Its objective is to protect the health and safety of people, and to protect the environment, by identifying risks posed by or as a result of gene technology, and by managing those risks through regulating certain dealings with genetically modified organisms (GMOs). This chapter describes the context within which potential risks to the health and safety of people or the environment posed by the proposed release are assessed. The risk assessment context is established within the regulatory framework and considers application-specific parameters (Figure 1). RISK ASSESSMENT CONTEXT LEGISLATIVE REQUIREMENTS (including Gene Technology Act and Regulations) RISK ANALYSIS FRAMEWORK OGTR OPERATIONAL POLICIES AND GUIDELINES PROPOSED DEALINGS Proposed activities involving the GMO Proposed limits of the release Proposed control measures GMO Introduced genes (genotype) Novel traits (phenotype) PREVIOUS RELEASES Figure 1. PARENT ORGANISM Origin and taxonomy Cultivation and use Biological characterisation Ecology RECEIVING ENVIRONMENT Environmental conditions Agronomic practices Presence of related species Presence of similar genes Summary of parameters used to establish the risk assessment context Section 2 Regulatory framework Sections 50, 50A and 51 of the Act outline the matters which the Gene Technology Regulator (the Regulator) must take into account, and the consultation required when preparing the Risk Assessment and Risk Management Plans (RARMPs) that inform the decisions on licence applications. In addition, the Regulations outline further matters the Regulator must consider when preparing a RARMP. In accordance with section 50A of the Act, this application is considered to be a limited and controlled release application, as its principal purpose is to enable the applicant to conduct experiments and the applicant has proposed limits on the size, locations and duration of the release, as well as controls to restrict the spread and persistence of the GMOs and their genetic material in the environment. Therefore, the Regulator was not required to consult with prescribed experts, agencies and authorities before Chapter 1 – Risk assessment context 1 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator preparation of the Risk Assessment and Risk Management Plan (RARMP; see section 50 of the Act). Section 52 of the Act requires the Regulator to seek comment on the RARMP from the States and Territories, the Gene Technology Technical Advisory Committee, Commonwealth authorities or agencies prescribed in the Regulations, the Minister for the Environment, relevant local council(s), and the public. The advice from the prescribed experts, agencies and authorities and how it was taken into account is summarised in Appendix A. Three public submissions were received and their considerations are summarised in Appendix B. The Risk Analysis Framework (OGTR 2013) explains the Regulator’s approach to the preparation of RARMPs in accordance with the legislative requirements. Additionally, there are a number of operational policies and guidelines developed by the Office of the Gene Technology Regulator (OGTR) that are relevant to DIR licences. These documents are available from the OGTR website. Any dealings conducted under a licence issued by the Regulator may also be subject to regulation by other Australian government agencies that regulate GMOs or GM products, including Food Standards Australia New Zealand (FSANZ), Australian Pesticides and Veterinary Medicines Authority (APVMA), Therapeutic Goods Administration, National Industrial Chemicals Notification and Assessment Scheme and Department of Agriculture. These dealings may also be subject to the operation of State legislation declaring areas to be GM, GM free, or both, for marketing purposes. Section 3 The proposed dealings The Commonwealth Scientific and Industrial Research Organisation (CSIRO) proposes to release up to 180 lines3 of genetically modified (GM) safflower into the environment under limited and controlled conditions. The purpose of the trial is to evaluate the agronomic performance and oil content of the GM safflower lines under field conditions in various regions around Australia. Oil generated from the trial would be used for product development and testing in industrial processes. The trial would also generate data to be used in future regulatory submissions. The dealings involved in the proposed intentional release include: conducting experiments with the GMOs breeding the GMOs propagating the GMOs growing or culturing the GMOs transporting the GMOs disposing of the GMOs and the possession, supply or use of the GMOs for the purposes of, or in the course of, any of the above. These dealings are detailed further below. 3.1 The proposed limits of the dealings (size, locations, duration and people) The applicant proposes to grow GM safflower plants over four growing seasons from January 2015 to August 2019. The term ‘line’ is used to denote plants derived from a single plant containing a specific genetic modification resulting from a single transformation event. 3 Chapter 1 – Risk assessment context 2 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator In the first year, 2015, up to 10 sites of 5 hectares (ha), in the second and third years (2016, 2017) up to 15 sites of 10 ha each per year, and in the final year (2018) up to 20 sites of 25 ha each will be grown. The total maximum planting area is 850 ha over the period of the trial. The GMOs are proposed to be planted at up to 604 sites across Australia. Sites may be located in any of 52 local government areas (LGAs) in New South Wales, 36 LGAs in Victoria, 4 LGAs in Western Australia, 16 LGAs in Queensland and 1 LGA in the Australian Capital Territory, as listed in Table 1. The exact sites will be determined closer to planting and their selection will depend on a number of factors including: adequate site distribution across Australian safflower growing areas; the ability to ensure isolation and containment; and the ability to segregate from commercial safflower crops. Table 1. Proposed local government areas in which GM safflower may be released. New South Wales Berrigan Orange Bland Parkes Blayney Tamworth Boorowa Temora Cabonne Tumbarumba Conargo Tumut Coolamon Upper Hunter Coonamble Urana Cootamundra Wagga Wagga Corowa Wakool Cowra Walgett Deniliquin Warren Dubbo Warrumbungle Forbes Weddin Gilgandra Wellington Greater Hume Young Griffith Gunnedah Gundagai Gwydir Harden Hay Inverell Jerilderie Junee Leeton Liverpool Plains Lockhart Mid-Western Moree Plains Murray Murrumbidgee Muswellbrook Narrabri Narrandera Narromine 4 Queensland Burdekin Central Regional Highlands Mackay Maranoa Regional North Burnett Regional South Burnett Regional Toowoomba Regional Southern Downs Regional Western Downs Regional Goondiwindi Regional Balonne Moreton Bay Bundaberg Cairns Hinchinbrook Bowen Western Australia Kununurra Mt Barker Merredin Katanning Australian Capital Territory Ginninderra Victoria Ararat Ballarat Benalla Buloke Greater Bendigo Campaspe Central Goldfields Colac Otway Corangamite Gannawarra Glenelg Golden Plains Greater Geelong Greater Shepparton Hepburn Hindmarsh Horsham Indigo Loddon Macedon Ranges Mildura Mitchell Moira Moorabool Mount Alexander Moyne Northern Grampians Pyrenees Southern Grampians Strathbogie Swan Hill Wangaratta West Wimmera Wodonga Wyndham Yarriambiack During consultation the total number of sites was clarified as 60, not 45 as reported in consultation RARMP Chapter 1 – Risk assessment context 3 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator The applicant is proposing that only trained and authorised staff would be permitted to deal with the GM safflower. Any visitors to the site would be accompanied by an authorised CSIRO representative and would not deal with the GMOs. 3.2 The proposed controls to restrict the spread and persistence of the GMOs and their genetic material in the environment The applicant has proposed a number of controls to restrict the spread and persistence of the GM safflower plants and the introduced genetic material in the environment. These include: locating the trial sites at least 50 m from natural waterways ensuring that no other safflower crops are grown within 400 m (Exclusion Zone) of the trial sites surrounding each site with a 10 m Monitoring Zone kept free of weeds or related species surrounding the Monitoring Zone with an Isolation Zone of 190 m, or 50 m if no safflower or related species are observed during the previous season, within which safflower plants and related species will be controlled restricting bird access by bird netting, bird scarers or growing a sacrificial crop attractive to birds (such as sorghum) as appropriate to local site conditions monitoring for and controlling rodents at trial sites harvesting GM safflower separately to other crops or trials cleaning equipment prior to removal from the site destroying all plant materials not required for testing or future trials promoting germination of residual seed by post-harvest tillage and irrigation post-harvest monitoring of the trial sites for at least two years and destruction of any volunteer safflower transporting and storing GM material in accordance with the Regulator’s Guidelines for the Transport, Storage and Disposal of GMOs not allowing GM plant material or products to be used for human food or animal feed. Figure 2 shows the proposed site layout, including some of these controls. These controls, and the limits outlined above, have been taken into account in establishing the risk assessment context (this Chapter). Their suitability for containing the proposed release is evaluated in Chapter 3, Section 3.1.1. Chapter 1 – Risk assessment context 4 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) A 190 m Isolation Zone. Office of the Gene Technology Regulator An Inspection Zone occupies all or part (50 m) of the Isolation Zone and is inspected for the presence of Safflower and Related Species. A 10 m Monitoring Zone which is kept free of safflower and related species high. A 400 m Exclusion Zone where no other Safflower crops may be grown. Figure 2. Planting Area where GM and non-GM safflower are planted Proposed trial layout, including some of the controls (not drawn to scale) Section 4 The parent organism The parent organism of the GMOs is safflower (Carthamus tinctorius L.), a member of the Asteraceae family. Safflower is exotic to Australia and is cultivated as an annual oilseed crop. It is a branching thistle-like herbaceous plant with spiny leaves (Singh & Nimbkar 2006). Detailed information about the parent organism is contained in the reference document The Biology of Carthamus tinctorius L. (safflower)(OGTR 2015), which was produced to inform the risk assessment process for licence applications involving GM safflower. This document is available from the OGTR website. Some of the information from this document is summarised below. Safflower has been commercially cultivated as a minor crop in Australia since the 1950s. The growing area of safflower has fluctuated from year to year, with a peak of 75,000 hectares in 1979, which is less than 0.5% of the total cropping area in Australia. Over the past decade the average annual safflower planting area has ranged from 6,000-12,000 ha, this being mainly in New South Wales, Victoria and South Australia (ABARES 2014). It is grown in Australia for the edible oil and industrial oil markets, but also whole safflower seeds are used for the birdseed market (GRDC 2010). After oil is extracted from the seeds, the remaining meal can be used as stockfeed. Cultivars of safflower are divided into two main classes. Linoleic safflower varieties have oil rich in linoleic acid (70-75%), while oleic safflower varieties have oil with high levels of oleic acid (70-75%) (Singh & Nimbkar 2006). The GM safflower lines proposed for release were derived from four elite oleic safflower selections developed from a commercially available cultivar from Mexico, CIANOL-OL. CIANOL-OL was developed from the widely used cultivar S317. Neither of these cultivars are currently grown in Australia. Safflower is highly adaptable to different regions and safflower production extends from southern Canada (about 500N) to southern Australia (about 400S). It is generally planted in the winter or early spring in Australia. Safflower is relatively drought tolerant due to its extensive tap root system that can access moisture from deep in the soil profile. Safflower does have a relatively high water requirement but does not tolerate waterlogging, as this encourages development of the fungal diseases Alternaria carthami and Phytophthora species. Safflower seedlings at the rosette stage are resistant to cold and frosts as low as -7°C, but during stem Chapter 1 – Risk assessment context 5 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator elongation the growing point and stem can be damaged or killed by frosts below -4⁰C. Mean daily temperatures above 26⁰C during flowering and maturation reduce yield. Safflower is fairly slow-growing with a period of 18-31 weeks between sowing and maturity, depending on cultivar, sowing time and weather conditions (GRDC 2010). Safflower is either self-pollinated or insect pollinated and its pollen is not transported appreciably by wind beyond 1 m (Claassen 1950). Many safflower varieties are 85-90% selfpollinating with insects, primarily honey bees, responsible for the remaining 10-15% (USDAAPHIS 2008). Outcrossing rates between safflower plants in close proximity (1-1.5 m) appear to be highly variable and can range from 0-100% (Claassen 1950) with an average outcrossing rate of 10% (GRDC 2010). Long distance outcrossing between safflower plants has been reported in a single experiment to occur at a rate of 0.12% and 0.01% at 50 to 100 m, respectively (McPherson et al. 2009a). Safflower reproduces by seeds, which are smooth and fairly large, 6-7 mm, each weighing approximately 40 mg (GRDC 2010). The seed heads are highly resistant to shattering. Safflower seeds have very low dormancy and ripe seeds may germinate in the head following rainfall. The low dormancy rates means that seeds that fall to the ground during harvest are expected to germinate readily. The little seed dormancy reported in safflower is lost during storage. Viable safflower seed persistence in the seed bank is less than two years at the soil surface and less than one year if the seeds are buried in the soil (McPherson et al. 2009b). Animal predation of safflower is limited due to its spiny nature. Bird predation of safflower seed occurs, but studies of some bird species (blackbirds, mallard ducks, pheasants & pigeons) show that seeds that have passed through the digestive systems are no longer viable (Cummings et al. 2008; GRDC 2010). Industry standards suggest an isolation distance of 400 m for producing basic safflower seed, which is planted to produce certified seed (OECD 2013). Safflower seed oil and the seed meal are generally not considered to be toxic and have a long history of safe use. However, anti-nutrient compounds such as lignan glucosides and tannins and natural toxins such as hydrogen cyanide and oxalates are present in the seed (Ingale & Shrivastava 2011; Kuehnl et al. 2013). These anti-nutrient compounds and toxins are present in such low amounts that the safflower meal does not appear to be toxic when fed to animals. High fibre content of the safflower seed or seed meal is the main factor limiting its use in livestock feed. Safflower petal extracts have been used in Chinese herbal medicine for centuries and there are many reports on the beneficial effects of safflower in the treatment of several conditions (Chengaiah et al. 2010; Zhou et al. 2014). Rare cases of allergic reactions to safflower dried flowers have been reported (Compes et al. 2006). There are also reports of adverse effects of flower extracts examined in animal studies indicating potential teratogenicity (Monfared 2013; Nobakht et al. 2000), cytotoxicity (Mohseni et al. 2011) and nephrotoxicity (Liu et al. 2004). Section 5 The GMOs, nature and effect of the genetic modification 5.1 Introduction to the GMOs The applicant proposes to release up to 180 lines of GM safflower. All lines were produced by Agrobacterium tumefaciens-mediated plant transformation. Information about this transformation method can be found in the risk assessment reference document Methods of plant genetic modification available from the Risk Assessment References page on the OGTR website. Details of the techniques used specifically for transformation of safflower are described elsewhere (Belide et al. 2011). There are nine categories of GM safflower proposed for release with each category having 20 lines. The lines contain introduced gene silencing constructs containing fragments of Chapter 1 – Risk assessment context 6 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator either two or three endogenous safflower fatty acid biosynthesis genes. The categories differ in the regulatory genetic elements used in the RNAi vectors or constructs. The function of the silencing constructs is to suppress the expression of the corresponding (target) fatty acid biosynthesis genes, and thus alter the oil composition of the GM safflower seeds. Specifically, the content of the fatty acid oleic acid should be increased. 5.2 The introduced genes, encoded proteins and their associated effects The three safflower genes that are targeted for suppression of expression are palmitoyl-ACP thioesterase (FATB), Δ12 desaturase (FAD2) and another fatty acid biosynthesis gene. The identity of this third gene, promoters and molecular details of the silencing constructs were declared CCI in relation to a previous GM safflower application (DIR 121). Three additional regulatory genetic elements have been declared as CCI in relation to the DIR 131 application. The confidential information was made available to the prescribed experts and agencies that were consulted on the RARMP for this application. Suppression of the target genes is mediated by using a natural regulatory mechanism in plants known as ribonucleic acid interference (RNAi) or gene silencing (Baykal & Zhang 2010). Using the RNAi pathway, an introduced silencing construct is transcribed into doublestranded RNA, which is processed by endogenous cellular machinery into short interfering RNAs (siRNAs). The siRNAs direct the degradation of messenger RNA (mRNA) molecules with matching sequence after the mRNAs are transcribed from genes and before they are translated into proteins. The efficiency of gene silencing is generally determined by the extent of homology between the silencing construct and the target gene (usually > 95% homology is required) and the length of the homologous region. In plants, introduced silencing constructs have been shown to effectively suppress expression of the target genes, but can also give rise to silencing of non-target genes with closely matching sequences. The target gene FATB encodes a carrier protein that mediates export of saturated fatty acids from the plastid, where fatty acid synthesis occurs (Bonaventure et al. 2003). The effect of suppressing expression of FATB is to retain saturated fatty acids in the plastid until they undergo a desaturation reaction, usually to form oleic acid, and can be exported by another carrier protein. This decreases the proportion of saturated fatty acids and increases the proportion of oleic acid in the safflower oil. The target gene FAD2 encodes a desaturase protein that mediates enzymatic conversion of oleic acid to linoleic acid (Harwood 1996). The effect of suppressing expression of FAD2 is to decrease the proportion of linoleic acid and increase the proportion of oleic acid in the safflower oil. The GM safflower lines produce seeds where 90-95% of the total oil content is oleic acid. This high purity oleic oil has potential application as an industrial raw material and a replacement to petroleum-based oils in the manufacture of plastics, lubricants and cosmetics (Vanhercke et al. 2013). All of the GM safflower lines also contain the introduced hph gene which provides resistance to the antibiotic hygromycin B, and is used as a selectable marker during plant transformation. This gene is derived from E. coli and truncated for use in plants. It is expressed under the control of either the enhanced tobacco constitutive ubiquitous promoter (enTCUP) or the constitutive 35S promoter from Cauliflower mosaic virus (CaMV) and the nopaline synthase (nos) terminator from A. tumefaciens. Some of the GM safflower lines also contain the introduced gfp gene, which encodes a green fluorescent protein used to visually identify genetically modified plant cells. This gene is derived from the jellyfish Aequorea victoria. It is expressed under the control of the CaMV 35S promoter and the octopine synthase (ocs) terminator from A. tumefaciens. Chapter 1 – Risk assessment context 7 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator The hph and gfp marker genes are commonly used in gene technology. Further details about these genes can be found in the risk assessment reference document Marker genes in GM plants available from the Risk Assessment References page on the OGTR website. 5.3 Toxicity/allergenicity associated with the introduced safflower genes Insertion of safflower gene fragments as part of gene silencing constructs does not result in expression of a protein, but only in suppression of the expression of endogenous safflower proteins. This is not expected to lead to increased toxicity or allergenicity. The effect of the gene silencing is to increase levels of oleic acid and decrease levels of other fatty acids in GM safflower seed oil. Oleic acid is a common constituent of food, for example, it is the main constituent of olive oil and canola oil. Oleic acid is associated with health benefits and it is not associated with toxicity or allergenicity. No studies on the toxicity or allergenicity of the GM safflower lines and their products have been undertaken. The GM safflower is not intended to be used as human food or animal feed. 5.4 Characterisation of the GMOs 5.4.1 Stability and molecular characterisation Transformation of the GM safflower lines was confirmed using both polymerase chain reaction (PCR) assays and analysis of oil content. Lines were self-pollinated and selected through single seed descent for between 2-5 generations. Standard Mendelian inheritance of the introduced genetic material was observed. The copy numbers of the introduced genetic material were determined by Southern blot hybridisation for only a few of the GM lines proposed for release. Released lines will contain one or more insertions. The genomic locations of the introduced genetic material have not been characterised for any of the GM lines. Phenotypic characterisation Some GM safflower lines proposed for release under this application were grown in greenhouses under controlled conditions or in field trials under licence DIR 121. The same genes are targeted for silencing in both DIR 121 and DIR 131, but additional regulatory elements are included in this application. According to the applicant, no phenotypic differences between the GM safflower plants and non-GM plants have been observed. GM safflower lines had the same growth patterns, morphology and fertility as non-GM comparators. Additionally, the applicant indicated that no phenotypic differences were observed for a range of traits between non-GM safflower and GM safflower lines grown in the field under DIR 121. Traits that were examined included germination rate, time to flowering, height, seed count per plant and seed oil content. Section 6 The receiving environment The receiving environment includes: any relevant biotic/abiotic properties of the geographic regions where the field trial would occur; intended agricultural practices, including those that may be altered in relation to normal practices; other relevant GMOs already released; and any particularly vulnerable or susceptible entities that may be specifically affected by the proposed release (OGTR 2013). The proposed dealings involve planting the GM safflower at up to 60 sites throughout Australia. Proposed local government areas (LGAs) for release sites are listed in Table 1, Section 3.1. Sites may be located in any of 16 LGAs in Queensland, 52 LGAs in New South Wales, 36 LGAs in Victoria, 4 LGAs in Western Australia, and one in the Australian Capital Territory. The sites will only be used for safflower growing, seed production and storage. Chapter 1 – Risk assessment context 8 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator 6.1 Relevant abiotic factors The abiotic factors relevant to the growth, distribution and cultivation of commercial safflower in Australia can be found in Raising the Bar with Better Safflower Agronomy (GRDC 2010) and The Biology of Carthamus tinctorius L. (safflower) (OGTR 2015). The proposed release will be carried out across a range of geographic and climatic conditions. The applicant proposes to locate field trials at least 50 m away from natural waterways. 6.2 Relevant agricultural practices GM safflower seeds would be planted in trial sites in winter or early spring. The trial sites would include plots of GM safflower lines, non-GM parental safflower varieties, and nonGM commercial safflower varieties. Non-GM safflower grown at the trial sites would be treated as if it were GM safflower. The proposed 10 m monitoring zone surrounding each trial site may be either kept as bare fallow or planted with vegetation maintained at a height of less than 10 cm such as grass species, and would be kept free of weeds. The applicant proposes to harvest all GM safflower at maturity. The GMOs would be harvested separately from other crops. Any equipment used for harvesting or other operations would be cleaned on-site prior to removal or use for any other purpose. Fallen seed and non-propagative plant material remaining at the field locations after harvest would be ploughed into the ground or buried to a depth of 1m. The sites would be watered post-harvest to encourage seed germination and monitored for volunteers. Volunteers would be removed by hand or killed by herbicide application. 6.3 Presence of related plants in the receiving environment Safflower is grown as a minor commercial crop in Australia. An average of 10,000 hectares of safflower is grown annually in South Australia, New South Wales and Victoria (ABARES 2014). Many of the proposed trial sites around Australia are within current safflower growing areas and it is possible that non-GM safflower crops will be grown nearby. Naturalised populations of wild safflower have been reported at low levels in all states and territories of Australia (Atlas of Living Australia, www.ala.org.au). Wild safflower is considered a minor weed that primarily establishes on disturbed ground (Groves et al. 2003).Wild safflower has been classified as a category 1 weed of agricultural ecosystems specifically in Queensland, Northern Territory and South Australia; it was present but not rated as a weed in New South Wales or Victoria. Wild safflower has been rated as a category 3 weed of natural ecosystems in Australia, a category 3 weed is naturalised and known to be a minor problem warranting control at 4 or more locations within a State or Territory (Groves et al. 2003). There are four related Carthamus species reported as present in Australia: C. lanatus, C. leucocaulos, C. dentatus and C. glaucus. All four species have a chromosome number of n=10, whereas for safflower n=12. These related species have all been reported as naturalised in Australia (Atlas of Living Australia). Both C. lanatus and C. leucocaulos have been declared noxious weeds in some states or territories (Weeds Australia). There are doubts about the existence of C. glaucus in Australia; the two specimens that formed the basis of the record of this species in the 1986 Flora of SA have now been re-determined as C. leucocaulos, and the same may have happened in other States (personal communication Micheala Heinson, PIRSA, SA government). Under controlled conditions, C. leucocaulos and C. lanatus can cross with C. tinctorius but produce sterile F1 hybrid plants (Mayerhofer et al. 2011). One study of crosses between C. tinctorius and C. glaucus produced fertile offspring under controlled conditions, but doubts have been raised about the identity of C. glaucus seeds supplied (Mayerhofer et al. Chapter 1 – Risk assessment context 9 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator 2011), whereas another study indicated hybrids with C. glaucus are sterile (Ashri & Knowles 1960). Similar to the other n=10 species above, formation of viable hybrids between C. dentatus and safflower (n = 12) is unlikely due to different chromosome numbers (see reviews by Kumar, 1991 and McPherson et al. 2004). 6.4 Presence of similar genes and encoded proteins in the environment The three gene fragments included in the silencing constructs are from endogenous safflower genes that are naturally present in all safflower plants. The hph antibiotic resistance gene is from E. coli, which is widespread and prevalent in the environment, including in the human and animal digestive systems. The gfp gene is from jellyfish and exposure to the GFP protein in the terrestrial environment would be unlikely. Regulatory sequences are derived from common plants, plant viruses (CaMV) or a soil bacterium (Agrobacterium tumefaciens) that are widespread in the environment. Although some of the regulatory sequences are derived from plant pathogens (A. tumefaciens and CaMV), they comprise only small parts of the total genomes and cannot of themselves cause disease. Section 7 Relevant Australian and international approvals 7.1 Australian approvals 7.1.1 Approvals by the Regulator The Regulator has approved field trials of GM safflower lines with the trait of increased oleic acid under licence DIR 121. The Regulator has previously approved field trials of GM cotton with the trait of increased levels of oleic acid under licences DIR 039/2003 and DIR 085/2008. Information on these licences is available from the GMO Record on the OGTR website. There have been no reports of adverse effects on human health or the environment resulting from any of these releases. 7.1.2 Approvals by other government agencies FSANZ has previously approved food derived from lines of GM soybean with the trait of increased levels of oleic acid as safe for human consumption (FSANZ 2009; FSANZ 2011). 7.2 International approvals None of the GM safflower lines proposed for release in this application have been approved for release in other countries. Field trials of different GM safflower lines, with various introduced traits, have been approved in the United States and Canada (CFIA 2015; USDA-APHIS 2008). Chapter 1 – Risk assessment context 10 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Chapter 2 Section 1 Office of the Gene Technology Regulator Risk assessment Introduction The risk assessment identifies and characterises risks to the health and safety of people or to the environment from dealings with GMOs, posed by or as the result of gene technology (Figure 3). Risks are identified within the context established for the risk assessment (see chapter 1), taking into account current scientific and technical knowledge. A consideration of uncertainty, in particular knowledge gaps, occurs throughout the risk assessment process. Figure 3. The risk assessment process Initially, risk identification considers a wide range of circumstances whereby the GMO, or the introduced genetic material, could come into contact with people or the environment. Consideration of these circumstances leads to postulating plausible causal or exposure pathways that may give rise to harm for people or the environment from dealings with a GMO (risk scenarios) in the short and long term. Postulated risk scenarios are screened to identify substantive risks that warrant detailed characterisation. A substantive risk is only identified for further assessment when a risk scenario is considered to have some reasonable chance of causing harm. Pathways that do not lead to harm, or could not plausibly occur, do not advance in the risk assessment process. A number of risk identification techniques are used by the Regulator and staff of the OGTR, including checklists, brainstorming, reported international experience and consultation (OGTR 2013). A weed risk assessment approach is used to identify traits that may contribute to risks from GM plants. In particular, novel traits that may increase the potential of the GMO to spread and persist in the environment or increase the level of potential harm compared with the parental plant(s) are used to postulate risk scenarios (Keese et al. 2014). In addition, risk scenarios postulated in previous RARMPs prepared for licence applications of the same and similar GMOs are also considered. Substantive risks (i.e. those identified for further assessment) are characterised in terms of the potential seriousness of harm (Consequence assessment) and the likelihood of harm (Likelihood Chapter 2 – Risk assessment 11 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator assessment). The level of risk is then estimated from a combination of the Consequence and Likelihood assessments. Risk evaluation then combines the Consequence and Likelihood assessments to determine level of risk and whether risk treatment measures are required. The potential for interactions between risks is also considered. Section 2 Risk Identification Postulated risk scenarios are comprised of three components (Figure 4) i. The source of potential harm (risk source). ii. A plausible causal linkage to potential harm (causal pathway). iii. Potential harm to an object of value, people or the environment. Figure 4. Risk scenario In addition, the following factors are taken into account when postulating relevant risk scenarios: the proposed dealings, which may be to conduct experiments, develop, produce, breed, propagate, grow, import, transport or dispose of the GMOs, use the GMOs in the course of manufacture of a thing that is not the GMO, and the possession, supply and use of the GMOs in the course of any of these dealings the proposed limits including the extent and scale of the proposed dealings the proposed controls to limit the spread and persistence of the GMO characteristics of the parent organism(s). Additional information relevant to the risk assessment has been declared CCI by the Regulator (either under DIR 121 or DIR 131) and was made available to the prescribed experts and agencies that were consulted on the RARMP for this application. 2.1 Risk source The source of potential harms can be intended novel GM traits associated with one or more introduced genetic elements, or unintended effects/traits arising from the use of gene technology. As discussed in Chapter 1, the GM safflower proposed for release will be modified by the introduction of gene silencing constructs containing fragments of either two or three endogenous safflower genes designed to induce the suppression of fatty acid biosynthesis genes. These introduced gene constructs are considered further as potential sources of risk. There are nine categories of GM safflower proposed for release with 20 lines in each category. All the GM lines contain the hph hygromycin tolerance selection marker gene and lines from categories 1 to 3 contain the gfp visual marker gene (see Chapter 1). However, these marker genes and their products have already been considered in detail in previous RARMPs (for example DIR 077/2007 for hph and DIR 096/2009 for gfp) and assessed as posing negligible risk to human or animal health or to the environment by the Regulator. As these genes have not been found to pose substantive risks to either people or the environment, their potential effects will not be further Chapter 2 – Risk assessment 12 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator assessed for this application. Further information about these genes can be found in the document Marker genes in GM plants available from the Risk Assessment References page on the OGTR website. All of the introduced gene constructs contain regulatory sequences that are necessary for the desired expression characteristics of the silencing sequences and the selection marker genes. These regulatory sequences come from A. tumefaciens, CaMV, A. thaliana, N. tabacum, and R. communis. Specific details of the regulatory sequences have been declared CCI by the Regulator (either under DIR 121 or DIR 131) and were made available to the prescribed experts and agencies that were consulted on the RARMP for this application. There is no evidence that regulatory sequences themselves have toxic or allergenic effects (EPA 1996); such effects for these sequences will not be further assessed for this application. Although the viral sequence is derived from a plant pathogen, it only constitutes a small part of the genome and cannot itself cause disease. However, regulatory sequences, especially promoters, control gene expression and hence the levels of the derived RNA molecules and proteins in the GM plants. The effects of these levels of these molecules, in particular if they affect the toxicity and allergenicity of the GM plants and their interaction in the environment, will be discussed below. 2.2 Causal pathway The following factors are taken into account when postulating plausible causal pathways to potential harm: routes of exposure to the GMOs, the introduced gene(s) and gene product(s) potential effects of the introduced gene(s) and gene product(s) on the properties of the organism potential exposure to the introduced gene(s) and gene product(s) from other sources in the environment the environment at the site(s) of release agronomic management practices for the GMOs spread and persistence (invasiveness) of the GM plant, including o establishment o reproduction dispersal by natural means and by people tolerance to abiotic conditions (e.g. climate, soil and rainfall patterns) tolerance to biotic stressors (e.g. pest, pathogens and weeds) tolerance to cultivation management practices gene transfer to sexually compatible organisms gene transfer by horizontal gene transfer (HGT) unauthorised activities. Although all of these factors are taken into account, some have been considered in previous RARMPs or are not expected to give rise to substantive risks. The potential for horizontal gene transfer (HGT) and any possible adverse outcomes has been reviewed in the literature (Keese 2008) as well as assessed in many previous RARMPs. These RARMPs are available from the GMO Record on the OGTR website or by contacting the OGTR. No risk greater than negligible was identified due to the rarity of these events, relative to those HGT events that occur in nature, and the limited chance of providing a selective advantage to the recipient organism that would promote the spread and persistence of the transferred material. Therefore, HGT will not be assessed further. Chapter 2 – Risk assessment 13 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator The potential for unauthorised activities to lead to an adverse outcome has been considered in previous RARMPs. The Act provides for substantial penalties for non-compliance and unauthorised dealings with GMOs. The Act also requires the Regulator to have regard to the suitability of the applicant to hold a licence prior to the issuing of a licence. These legislative provisions are considered sufficient to minimise risks from unauthorised activities, and no risk greater than negligible was identified in previous RARMPs. Therefore, unauthorised activities will not be considered further. 2.3 Potential harm Potential harms from GM plants include: harm to the health of people or desirable organisms, including toxicity/allergenicity reduced establishment of desirable plants, including having an advantage in comparison to related plants reduced yield of desirable vegetation reduced products or services from the land use restricted movement of people, animals, vehicles, machinery and/or water reduced quality of the biotic environment (e.g. providing food or shelter for pests or pathogens) or abiotic environment (e.g. negative effects on fire regimes, nutrient levels, soil salinity, soil stability or soil water table) reduced biodiversity through harm to other organisms or ecosystems. These harms are based on those used to assess risk from weeds (Standards Australia New Zealand & CRC for Australian Weed Management 2006). Judgements of what is considered harm depend on the management objectives of the land where the GM plant is expected to spread and persist. A plant species may have different weed risk potential in different land uses such as dryland cropping or nature conservation. 2.4 Postulated risk scenarios Three risk scenarios were postulated and screened to identify substantive risk. These scenarios are summarised in Table 3 and more detail of these scenarios is provided later in this Section. Postulation of risk scenarios considers impacts of the GM safflower or its products on people undertaking the dealings, as well as impacts on people and the environment if the GM plants or genetic material were to spread and/or persist. In the context of the activities proposed by the applicant and considering both the short and long term, none of the three risk scenarios gave rise to any substantive risks. Chapter 2 – Risk assessment 14 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Table 3 Summary of risk scenarios from dealings with GM safflower genetically modified for high oleic acid composition Risk Risk source scenario 1 Introduced gene constructs affecting oleic acid content Causal pathway Potential harm Growing GM plants at the site Expression of introduced genes in GM plants Contact with GM plant material by people who work with the GMOs, or by other organisms at the trial sites Allergic reactions in people or toxicity in people and other organisms Substantive risk? No 2 Introduced gene constructs affecting oleic acid content Dispersal of GM seed outside trial limits Growth of GM plants Expression of introduced genes in GM plants Spread and persistence of populations of GM plants outside trial limits Exposure of people or other organisms to GM plant material Allergic reactions in people or toxicity in people and other organisms Reduced establishment or yield of desirable plants Reduced biodiversity No 3 Introduced gene constructs affecting oleic acid content Dispersal of GM pollen outside trial limits Vertical transfer of introduced genes to other sexually compatible plants (e.g. weedy relatives or commercial varieties of safflower) Expression of introduced genes in plants Spread and persistence of populations of GM plants outside trial limits Exposure of people or other organisms to GM plant material Allergic reactions in people or toxicity in people and other organisms Reduced establishment or yield of desirable plants Reduced biodiversity No Chapter 2 – Risk assessment Reason Insertion of the silencing constructs does not lead to expression of a protein. The biosynthetic pathway that is the target of the genetic modification does not produce known toxins or allergens. The proposed limits and controls, including not using the GM plant material for human food or animal feed, minimise exposure of people and other organisms to the GM plant. Insertion of the silencing constructs does not lead to the expression of a protein. The biosynthetic pathway that is the target of the genetic modification does not produce known toxins or allergens. The genetic modifications are not expected to increase the ability of the GM plants to spread and persist. The proposed limits and controls minimise the likelihood that GM plant material would leave trial limits. Safflower does not produce fertile hybrids with related weedy species in Australia. The introduced genes are not expected to increase the ability of hybrid GM plants to spread and persist or to be toxic or allergenic. The proposed limits and controls minimise the likelihood that GM pollen would be transferred to other plants 15 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) 2.4.1 Office of the Gene Technology Regulator Risk scenario 1 Risk source Introduced gene constructs affecting oleic acid content Causal pathway Growing GM plants at the site Expression of introduced genes in GM plants Contact with GM plant material by people who work with the GMOs, or by other organisms at the trial sites Potential harm Allergic reactions in people or toxicity in people and other organisms Risk source The source of potential harm for this postulated risk scenario is the introduced silencing constructs with fragments of safflower genes. Causal pathway The silencing constructs with safflower gene fragments are designed to produce siRNAs that suppress the expression of fatty acid biosynthetic genes, thus altering seed oil fatty acid content. A range of organisms may be exposed directly or indirectly to the introduced genetic constructs or their end products. Workers cultivating the GM safflower would be exposed to all plant parts. People who are involved in the breeding, cultivating, harvesting, transporting and processing of the GM safflower may be exposed to its products through contact (including inhalation of pollen). This would be expected to mainly occur in the trial site, but could also occur anywhere the GM plant material was transported or used for experimental analysis. Organisms, including birds, rodents and invertebrates, may be exposed directly to GM safflower plants through biotic interactions (vertebrates, invertebrates, symbiotic and/or pathogenic microorganisms), or through contact with dead plant material (soil biota) or indirectly through the food chain. The proposed limits and controls of the trial would minimise the likelihood that people or other organisms would be exposed to GM plant material. Although people dealing with the GMOs may directly handle the GM plant material, there is little potential for human ingestion of the GM safflower, as no GM plant material would be used as food. Human contact with GM plant materials would be limited to people with access to trial sites. The applicant proposes that GM plant materials will only be handled by trained and authorised staff. Similarly, livestock would not be intentionally exposed to the GMOs at the sites as the GM plant material is not to be used as animal feed. The spiny nature of safflower means that larger animals or livestock do not generally graze safflower. Rodent control measures will be used to reduce the number of rodents and appropriate bird control measures will be employed at each site to discourage birds from feeding on GM safflower. Further information about the GMOs relevant to consideration of the risk scenario has been declared CCI by the Regulator (either under DIR 121 or DIR 131) and was made available to the prescribed experts and agencies that were consulted on the RARMP for this application. Potential harm Toxicity is the adverse effect(s) of exposure to a dose of a substance as a result of direct cellular or tissue injury, or through the inhibition of normal physiological processes (Felsot 2000). Allergenicity is the potential of a substance to elicit an immunological reaction following its ingestion, dermal contact or inhalation, which may lead to tissue inflammation and organ dysfunction (Arts et al. 2006). The introduced gene silencing construct could lead to production of substances in the GM safflower that are toxic or allergenic for people or be toxic to other organisms. Transcription of the gene fragments in the silencing constructs forms hairpin RNA. This double-stranded RNA enters the RNAi pathway rather than being translated into a protein. Therefore, the introduction of the silencing constructs does not lead to expression of a novel protein that could potentially be toxic or allergenic. All known food allergens are proteins, those derived from plants coming chiefly from Chapter 2 – Risk assessment 16 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator peanut, tree nuts, wheat and soybean (Delaney et al. 2008; Herman & Ladics 2011). In these circumstances, there is no reasonable expectation that the introduced constructs will lead to an increase in the level of any endogenous compound in the GM safflower that has toxic or allergenic properties. Humans and animals have a long history of safe exposure to non-GM safflower. There is only one reported case of an allergenic reaction to safflower dried flowers in humans (Compes et al. 2006) and there are some reports of adverse toxic effects of safflower floral extract injections used in Chinese alternative medicine (see Chapter 1, Section 4). However, researchers indicated that adverse reactions may be due to other liposoluble components in the injections (Zhang et al. 2009). Safflower seed oil is non-allergenic and suitable for use in injectable medicines and cosmetics. By targeting fatty acid biosynthesis genes, the genetic modifications result in changed fatty acid profiles, specifically increasing the n-9 monounsaturated fatty acid oleic acid and decreasing other fatty acids such as the n-6-polyunsaturated fatty acid linoleic acid. There is uncertainty whether the genetic modification may result in changes to other fatty acids. In DIR 039/2003, high oleic GM-cotton was found to have elevated levels of two cyclopropenoid fatty acids, malvalic and sterculic acids. If changes do occur, it is expected to result in a decrease of fatty acids such as arachidonic acid or n-6 linoleic acid. The expected phenotypic difference between GM and non-GM safflower is that GM safflower oil will contain a higher proportion of oleic acid and a lower proportion of saturated (palmitic acid, stearic acid) or polyunsaturated (linoleic acid) fatty acids. However, oleic acid is part of the normal human diet, as it is a major constituent of vegetable oils and animal fats, and it is not considered toxic or allergenic. Hairpin RNA transcribed from the silencing constructs is processed into siRNAs. siRNAs fall under a general category of small RNAs that also includes microRNAs (miRNAs). siRNAs and miRNAs are common in both plants and animals and are believed to play regulatory roles in many biological processes. Animals and plants naturally produce thousands of different siRNA molecules and these are consumed by humans and other organisms whenever they eat plant or animal cells. One paper (Zhang et al. 2011) tracked the metabolic fate of a particular natural miRNA, miR-168a, that is produced abundantly in rice and other plants and happens to have a near perfect sequence match to a mammalian gene. In a study of mice fed a pure rice meal after fasting, the plant miRNA was detected in mouse livers and was reported to modulate the expression of the matching mammalian gene, reducing levels of the encoded protein in the liver by approximately 50%. The reported effect on the mouse gene by the plant miRNA was transient and ceased when rice was no longer included in the food intake. However, the quantity of rice fed to mice in the Zhang et al study (2011), which is equivalent to a human eating approximately 33 kg/day of cooked rice, is an unrealistic quantity in any human diet. A recent analysis paper (Petrick et al. 2013) suggests some potential alternate explanations for the findings of the Zhang et al study (2011), and after reviewing a number of other papers in the field concluded that the weight of the evidence does not suggest that miRNAs derived from normal dietary exposure have a meaningful effect on mammalian gene expression. However, a recent report indicated that another specific plant miRNA, miR-172, ingested by mice can survive the gastro-intestinal (GI) system and enter the bloodstream and various organs. In addition, miR-172 was detectable after what would be considered normal dietary exposure (i.e. equivalent to a human eating 280 g of the food source). The researchers did state that their results did not support general and consistent uptake of dietary plant miRNAs and additional studies are required to establish if plant miRNAs are transferred across the GI tract in sufficient quantity to regulate endogenous gene expression (Liang et al. 2014). The possibility exists that siRNAs produced in GM safflower lines could modulate expression of human or animal genes, with unknown physiological effects. The siRNAs would need to be produced at high levels in GM safflower, a large amount of the GM safflower would need to be consumed, the siRNA would need to match a target sequence in a human or animal gene, and be taken up by cells expressing that gene. As noted above, the GM safflower will not be used for human food or animal feed and the proposed limits and controls would minimise exposure of Chapter 2 – Risk assessment 17 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator people and other organisms to the GMOs. Mammals do not have genes that are homologous to the safflower fatty acid biosynthesis genes targeted by the introduced silencing constructs. Even if siRNAs were acquired through eating GM safflower and did affect expression of a mammalian gene, it is expected that any effect would be transient as described in Zhang et al (2011). The genetic modifications have the potential to cause unintended effects in the GM safflower plants in several ways including off-target siRNA-mediated silencing of genes expressed in safflower, altered expression of endogenous safflower genes by random insertion of introduced DNA in the genome, and secondary effects arising from altered substrate or product levels in biochemical pathways. Unintended effects might result in adverse outcomes such as toxicity or allergenicity. Unanticipated changes can also be induced in plants by conventional methods of plant breeding (Haslberger 2003). However, even though conventional breeding can involve the movement of hundreds and even thousands of genes into a plant, there has never been a report of a completely novel toxin or allergen appearing in a new line of a plant produced by such techniques (Steiner et al. 2013; Weber et al. 2012). The implication is that the movement into safflower of any of the genes that are the subject of this application, which themselves do not code for any proteins, is unlikely to result in the production (directly or indirectly) of a novel toxin or allergen. The range of possible unintended effects produced by genetic modification is not likely to be greater than that from accepted traditional breeding techniques (Bradford et al. 2005; Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human Health 2004). More detail on potential for unintended effects as a result the process of genetic modification can be found in the document Methods of plant genetic modification available from the Risk Assessment References page on the OGTR website. Conclusion: Risk scenario 1 is not identified as a substantive risk due to the introduced gene fragments not coding for any proteins, the biosynthetic pathway they will affect being involved in the production of compounds that have not been associated with toxic or allergenic reactions, and the proposed limits and controls are designed to minimise exposure of people and other organisms to the GM plant material. Therefore this risk could not be greater than negligible and does not warrant further detailed assessment. 2.4.2 Risk Scenario 2 Risk source Introduced gene constructs affecting oleic acid content Causal pathway Dispersal of GM seed outside trial limits Growth of GM plants Expression of introduced genes in GM plants Spread and persistence of populations of GM plants outside trial limits Exposure of people or other organisms to GM plant material Potential harm Allergic reactions in people or toxicity in people and other organisms Reduced establishment and yield of desirable plants, reduced biodiversity Risk source The source of potential harm for this postulated risk scenario is the introduced silencing constructs with fragments of safflower genes. Causal Pathway If GM safflower seed was dispersed outside the trial limits, this seed could germinate and give rise to GM plants expressing the introduced genes. These plants could spread and persist in the environment outside the trial limits and people and other organisms may be exposed to GM plant materials. Characteristics that influence the spread (dispersal of the plant or its genetic material) and persistence (establishment, survival and reproduction) of a plant species impact on the degree of its invasiveness. These characteristics include the ability to establish in competition with other plants, Chapter 2 – Risk assessment 18 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator to tolerate standard weed management practices, to reproduce quickly, prolifically and asexually as well as sexually, and to be dispersed over long distances by natural and/or human means. The degree of invasiveness of a plant species in a particular environment gives an indication of the likelihood of its weediness in that environment. In addition to local experience, a history of weediness overseas can be used as an indicator for weediness in Australia (Pheloung et al. 1999). Baseline information on the weediness of safflower is given in Chapter 1, Section 6, and more detailed consideration is available in The Biology of Carthamus tinctorius L. (safflower) (OGTR 2015). Safflower is fairly slow-growing, with an extended rosette stage following emergence and prior to stem development, during which it is poorly competitive with other plants (Dajue & Mündel 1996). Safflower plants are susceptible to a wide range of herbicides as well as physical weed management practices (GRDC 2010). Australia is the only country that currently classifies safflower as a weed (Groves et al. 2003). Potential dispersal of reproductive GM plant material outside the site boundaries would be limited to seed or pollen, as safflower does not reproduce vegetatively in the field. Safflower seed heads are resistant to shattering and the seeds lack seed dispersal characteristics such as stickiness, burrs and hooks, which can contribute to seed dispersal via animal fur or feathers. These seed dispersal characteristics are not expected to be altered in the GMOs. Gene flow via pollen is discussed in Section 2.4.3. Dispersal of viable seed could occur through a variety of ways including: endozoochory (dispersal through ingestion by animals), transport of seeds by animals, movement of seeds by people, or extremes of weather such as flooding or high winds. Trial sites would be located at least 50 m away from natural waterways to minimise seed dispersal in the event of flooding. Seeds dispersed by flooding would be unlikely to survive and establish, as safflower is very susceptible to damping off and fungal diseases in wet soil (GRDC 2010). Safflower is very resistant to shattering or lodging (Mündel et al. 2004), so seeds are unlikely to be dispersed by wind or via water runoff from irrigation or rainfall prior to harvest. Residual seeds that fall during harvest could be dispersed by water runoff from rainfall or by strong winds. However, the applicant proposes to till the trial sites post-harvest to incorporate GM plant material into the soil. Typical safflower seed losses during harvest are 3-4% (GRDC 2010) and the viability of these seed may range from 26 to 84% (McPherson et al. 2009b). Most of these seeds would germinate soon after harvest as safflower seeds have very low dormancy (see Chapter 1, Section 4). In a Canadian study, safflower seed did not persist beyond 2 years at the surface or 1 year when buried (McPherson et al. 2009b). Preliminary data from trials conducted under DIR 121 suggests that seed lost at harvest germinated within the first 2 months post-harvest with no further volunteers observed over the following seven months even though conditions were conducive for germination. It is not expected that the genetic modifications to safflower would affect seed yield, viability or germination. While the fatty acid composition is altered, the total fatty acid content of seeds, and thus their stored energy content, remains constant. GM safflower seeds grown in the greenhouse were reported to germinate and establish at the same rate as non-GM comparators. Likewise, it is not expected that the genetic modifications would affect the ability of the GMOs to survive the control measures being proposed, such as tilling or irrigating the trial sites and destroying any volunteers found during post-harvest monitoring. Small birds can feed on ripening safflower seed in the head, and cockatoos can chew off safflower plants at the base in order to access the seeds (GRDC 2010). Safflower seeds that have passed through the digestive systems of several bird species (blackbirds, mallard ducks, pigeons and pheasants) were observed to be no longer viable, but did remain viable in the oesophagus, crop and gizzard regions for several hours (Cummings et al. 2008). This study was on Northern Hemisphere bird species and results may differ for Australian bird species such as galahs, corellas or bush turkeys. The researchers also mentioned birds that hoard or cache seeds such as jays, crows and Chapter 2 – Risk assessment 19 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator ravens, as potential transport vectors of safflower seeds. It is not known whether Australian birds carry seeds away for later consumption. Safflower seeds did not attach to the plumage of the birds due to the smooth nature of safflower seeds, however, seeds were transported externally on soil attached to feet or legs of pigeons and pheasants (Cummings et al. 2008). The applicant proposes to prevent or control bird access to trial sites by using bird netting, bird scarers, or a sacrificial crop such as sorghum as a bird attractant, which would minimise seed dispersal through bird activity. A sorghum sacrificial crop was effective at deterring birds from feeding on GM safflower in the field under DIR 121. Large animals are generally deterred from grazing on standing safflower by its spines. Safflower seeds are firmly held within their seed heads, which limits their accessibility to rodents. Residual GM seeds post-harvest may attract animal predation, and could be transported and hoarded by rodents. However, the applicant proposes to till the trial sites post-harvest, which should bury the GM seeds. A 10 m monitoring zone around the trial sites would be monitored for rodents and maintained in a manner to minimise rodent activity. Rodents would be controlled if required. Hence, seed dispersal through animal or rodent activity is unlikely. Dispersal of seeds by people dealing with the GMOs would be minimised by cleaning of all equipment prior to removal from the trial sites. People/equipment could also disperse seed from the planting area to the adjacent monitoring zone during sowing or harvest. The applicant has proposed to inspect the monitoring zone for related species (including other safflower plants) and if found destroy them. Inspections would occur while the GMOs are growing and during post-harvest, thus, establishment and persistence of the GM safflower in the monitoring zone is unlikely. All GM plant material would be transported in accordance with the Regulator’s transport guidelines to avoid spillage. As discussed above, the proposed limits and controls of the trial would minimise the likelihood of spread and persistence of the GM plants and minimising potential for dispersal of seed and exposure to plant material by grazing livestock and people. Dispersal of GM plant material by authorised people entering the proposed trial site would be further minimised by a standard condition of DIR licences which requires the cleaning of all equipment used at the trial site. All plant materials not required for testing or future trials will be destroyed. Post-harvest tillage and irrigation is proposed to be used to encourage germination of any residual seed. The applicant proposes to monitor the trial sites for two years and destroy any volunteers prior to flowering. All GM plant material will be transported in accordance with the Regulator’s transport guidelines, which will minimise the opportunity for its dispersal. The effectiveness of the limits and controls are further discussed in Chapter 3. Potential harm As discussed in Section 2.3 of this Chapter, all plants have the potential to lead to harm in certain environments. For the purposes of this document, plant species causing significant levels of one or more of these harms are called “weeds”. A plant species may be weedy in one or more land uses, such as dryland cropping or nature conservation. As summarised in Chapter 1, Section 6.3, safflower is naturalised throughout Australia, primarily as an agricultural or ruderal weed and is classified as a category 1 weed in agricultural ecosystems or a category 3 weed in natural ecosystems in some States. Anecdotal evidence from safflower farmers (GRDC 2010) and weed risk assessment experts (personal communication, Stephen Johnson) in Australia indicate that safflower is not a significant weed in natural ecosystems. In reference to native habitats, there would have to be large numbers of GM plants before the establishment of native plants was affected. As discussed in risk scenario 1, the introduced gene products are not expected to be toxic to humans or other organisms and it is unlikely that the GM safflower plants would have higher toxicity and/or allergenicity than non-GM safflower. The only expected phenotypic difference Chapter 2 – Risk assessment 20 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator between GM safflower and non-GM safflower is altered fatty acid composition in the GM safflower oil. No phenotypic changes were observed between GM safflower and non-GM safflower grown in greenhouses or in the field under DIR 121, and a standard condition of a licence for a field trial would be that the applicant immediately notify the OGTR of any unintended effects. In the unlikely event of GM safflower plants establishing themselves beyond trial limits, this trait would not be expected to lead to populations of GM safflower that cause any environmental harms associated with weedy plants, such as reduced establishment or yield of desirable plants, or reduced biodiversity. Conclusion: Risk scenario 2 is not identified as a substantive risk due to the introduced gene fragments not coding for any proteins, the biosynthetic pathway they will affect being involved in the production of compounds that have not been associated with toxic or allergenic reactions, and the modified trait not being associated with weediness. Further, the proposed limits and controls are designed to minimise exposure of people and other organisms to the GM plant material and restrict seed dispersal. Therefore this risk could not be greater than negligible and does not warrant further detailed assessment. 2.4.3 Risk Scenario 3 Risk source Introduced gene constructs affecting oleic acid content Causal pathway Dispersal of GM pollen outside trial limits Vertical transfer of introduced genes to other sexually compatible plants, such as weedy relatives or commercial varieties of safflower Expression of genes in plants Spread and persistence of populations of GM plants outside trial limits Exposure of people or other organisms to GM plant material Potential harm Allergic reactions in people or toxicity in people and other organisms Reduced establishment and yield of desirable plants, reduced biodiversity Risk source The source of potential harm for this postulated risk scenario is the introduced silencing constructs with fragments of safflower genes. Causal pathway If the introduced silencing constructs were transferred and expressed in other safflower plants or related species, the resulting hybrid plants could have increased toxicity or allergenicity to people, toxicity to other organisms, or weediness potential. Vertical gene flow is the transfer of genetic information from an individual organism to its progeny by conventional heredity mechanisms, both asexual and sexual. In flowering plants, pollen dispersal is the main mode of gene flow (Waines & Hegde 2003). For GM crops, vertical gene flow could therefore occur via successful cross-pollination between the crop and neighbouring crops, plants, related weeds or native plants (Glover 2002). Alternatively, if seed was dispersed outside the trial site, GM plants may grow and subsequently disperse pollen. Hybrid plants possessing the introduced genes may form the basis for the spread of these genes in other varieties of safflower, or other sexually compatible plant species. As discussed in Chapter 1, there are four weedy Carthamus species that may be present in Australia: C. lanatus, C. leucocaulos, C. dentatus and C. glaucus. GM safflower could theoretically cross-pollinate plants from other Carthamus species at low levels if these weedy species were present in close proximity to the trial sites and flowered synchronously. If hybrids between GM safflower and a related Carthamus weedy species occurred, they would be annuals like all Carthamus species and are likely to be sterile (Mayerhofer et al. 2011). The hybrids could therefore only be transient weeds in the immediate environs of the trial sites, and could not lead to long-term Chapter 2 – Risk assessment 21 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator transfer of the introduced silencing constructs into weedy Carthamus species populations. As described in Chapter 1, Section 6.3, interspecific hybridisation between safflower and other species of the Carthamus genus present in Australia is difficult due to various cytogenetic barriers (e.g. varying chromosome number) and is unlikely to occur naturally as crosses have only been obtained under experimental conditions. The applicant has proposed to inspect the areas around the trial sites for the presence of sexually compatible plants. GM safflower could cross-pollinate non-GM safflower plants outside the trial if either naturalised safflower or commodity safflower crops were present in proximity to the trial sites. Safflower pollen is large and sticky and is not transported appreciably by wind beyond 1 m (Claassen 1950). Safflower varieties, including S317, are 85-90% self-pollinating with insects responsible for the remaining 10-15% (USDA-APHIS 2008). S317 is one of the parent varieties from which the varieties used in this trial have been developed. Honey bees are the primary species that mediate cross-pollination or outcrossing in safflower representing 70-85% of insect visitors to safflower fields (Boch 1961). The average foraging distance of honey bees from a colony is a few hundred metres in agricultural areas and generally honey bees do not forage past one mile (USDAAPHIS 2008). However, there is evidence of honey bees flying several kilometres between apiaries and safflower fields (Gary et al. 1977). In principle, GM safflower pollen could be widely dispersed, as bees forage over kilometre ranges. However, safflower pollen transported by an insect must compete with the floret’s own pollen to result in outcrossing. Bee-mediated cross-pollination of safflower may be less efficient than other crops, as transported safflower pollen is only reported to potentially fertilise the next floret visited by the bee (Cresswell 2010) in comparison to canola crops where pollen collected by a bee at one flower may fertilise up to twenty flowers visited subsequently (Cresswell et al. 2002). The reliable estimation of insect mediated gene flow in safflower is difficult and dependent on a number of factors including insect numbers and type, site layout and size, and distance between pollen donor and receptor (AOSCA 2012). Studies of outcrossing rates in safflower plants grown in close proximity show highly variable rates of outcrossing ranging from 0-50% (Nabloussi et al. 2013; Velasco et al. 2012) with average outcrossing rates of 10% and higher frequencies of 0-100% at the single plant and head level (Claassen 1950). Limited information is available on safflower cross-pollination over distance. For a commercial safflower cultivar studied in Canada, crosspollination rates were measured at 1.7% at 3 m, decreasing from 0.94 to 0.12% between 10 and 50 m, and again decreasing from 0.12 to 0.01% between 50 and 100 m, with no outcrossing observed at 300 m (McPherson et al. 2009a). This is the only study to determine safflower outcrossing at distances beyond a few metres. It should be noted that highly variable results were obtained for the three sites examined in this study, particularly the low outcrossing rates observed at one site which was suggested to be due to low or different pollinator populations (AOSCA 2012). In addition, the largest site examined was nine times the size of the other two sites and had four times the outcrossing rate (McPherson et al. 2009b). The OECD Seed Scheme for Varietal Certification, which applies in Australia and many other countries, requires that crops of certified safflower seed be grown with an exclusion distance of 200 m from other safflower crops, and that basic safflower seed (the source for certified seed crops) be grown with an exclusion distance of 400 m (OECD 2013). The AOSCA (2012) recommends an isolation distance of 1320ft (403 m) for producing certified safflower seed of all seed classes. For GM-safflower plants containing an introduced gene expressing a pharmaceutical product, the USDA-APHIS (2008) has required a 2 mile (3.2 km) exclusion distance between the GM crop and non-GM safflower crops, while in Canada an 800 m exclusion distance has been required (see CFIA website). These international guidelines were developed for conditions in the Northern Hemisphere where pollinators include both bumblebees and honeybees. Bumblebees are reported to be more effective at field-to-field pollination of safflower than honeybees (Cresswell 2010), so long-distance outcrossing rates may be reduced in mainland Australia compared to other countries due to the lack of bumblebees. However, this is unknown and in the North Hemisphere studies, bumblebees Chapter 2 – Risk assessment 22 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator represent less than 10% of insect visitors to safflower fields and in some studies bumblebees were not found to be present in safflower fields (Cresswell 1999; Cresswell 2000). The proposed limits and controls of the trial would minimise the likelihood of the dispersal of seed and exposure to GM plant material (as discussed in Risk Scenarios 1 and 2). The applicant proposes to inspect the monitoring and isolation zones, a distance of up to 200 m surrounding the trial sites, prior to flowering of GM safflower and to destroy any safflower plants and related species. This would minimise vertical gene flow to any safflower populations or related species within 200 m of the trial site. The applicant also proposes to ensure that no other safflower crops are grown within 400 m of the trial sites. The effectiveness of these control measures are discussed in Chapter 3. After completion of the trial, it is possible that whole GM plants could survive at the trial sites, or new volunteer plants could grow from residual seed in the trial sites. The applicant proposes a number of control measures to prevent these eventualities, including: destroying all plant materials not required for testing or future trials; promoting germination of residual seed by postharvest tillage and irrigation; and post-harvest monitoring of the trial sites for two years and destruction of any volunteer safflower prior to flowering. These measures would minimise potential for pollen flow from volunteer GM safflower plants following the trial. Potential harm If the vertical transfer of the introduced genes from the GM plants causes the recipient species to spread and persist in the environment to a degree greater than normally found amongst these species, they may produce one or more harms. People who are exposed to the GM plant material through contact or consumption of GM plant material may show toxic or allergenic reactions, while other organisms may show toxic reactions from consumption of GM plant material. The GM plants may act to reduce the establishment or yield of desired plants, and subsequently reduce biodiversity. In the rare event of the vertical transfer of the introduced genetic material from the GM plants to non-GM safflower plants or sexually compatible species, it is expected that this material in the recipient will have the properties that it possesses in the GM safflower parent. As discussed in risk scenario 1, the introduced genetic elements are not expected to result in GM plants that are allergenic to humans or toxic to humans or other organisms. Risk scenario 2 summarises the reasons that the introduced genetic elements are unlikely to make the GM safflower lines more weedy, these reasons being applicable to any plants to which the genes are transferred via hybridisation. Conclusion: Risk scenario 3 is not identified as a substantive risk due to the expected lack of toxicity, allergenicity or increased weediness in any offspring of the GM plants and other plants, either commercial safflower crops or other sexually compatible plants. Further, the proposed limits and controls are designed to minimise pollen dispersal. Therefore this risk could not be greater than negligible and does not warrant further detailed assessment. Section 3 Uncertainty Uncertainty is an intrinsic part of risk analysis5. There can be uncertainty about identifying the risk source, the causal linkage to harm, the type and degree of harm, the chance of harm occurring or the level of risk. In relation to risk management, there can be uncertainly about the effectiveness, efficiency and practicality of controls. Risk analysis can be considered as part of a first tier uncertainty analysis, namely a structured, transparent process to analyse and address uncertainty when identifying, characterising and evaluating risk. However, there is always some residual uncertainly that remains. If the residual uncertainty is important and critical to decision making, then this residual uncertainly may be 5 A more detailed discussion of uncertainty is contained in the Regulator’s Risk Analysis Framework available from the Risk Assessment References page on the OGTR website or via Free call 1800 181 030. Chapter 2 – Risk assessment 23 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator subjected to further analysis (= second tier uncertainly analysis), such as building ‘worst case’ scenarios, or by using meta-analysis where results from several studies are combined. There are several types of uncertainty in risk analysis (Bammer & SmithsonBammer & Smithson 2008; Clark & Brinkley 2001; Hayes 2004). These include: uncertainty about facts: – knowledge – data gaps, errors, small sample size, use of surrogate data – variability – inherent fluctuations or differences over time, space or group, associated with diversity and heterogeneity uncertainty about ideas: – description – expression of ideas with symbols, language or models can be subject to vagueness, ambiguity, context dependence, indeterminacy or under-specificity – perception – processing and interpreting risk is shaped by our mental processes and social/cultural circumstances, which vary between individuals and over time. For DIR 131, uncertainty is noted particularly in relation to the characterisation of: Potential for increased toxicity or allergenicity due to unintended effects Potential for increased weediness of the GMOs due to unintended phenotypic changes Potential for gene flow via pollen over long distance Additional data, including information to address these uncertainties, may be required to assess possible future applications for a larger scale trial, reduced containment conditions, or the commercial release of these GM safflower lines if they are selected for further development. Chapter 3, Section 4, discusses information that may be required for future release. Section 4 Risk Evaluation Risk is evaluated against the objective of protecting the health and safety of people and the environment to determine the level of concern and, subsequently, the need for controls to mitigate or reduce risk. Risk evaluation may also aid consideration of whether the proposed dealings should be authorised, need further assessment, or require collection of additional information. Factors used to determine which risks need treatment may include: risk criteria level of risk uncertainty associated with risk characterisation interactions between substantive risks. Three risk scenarios were postulated whereby the proposed dealings might give rise to harm to people or the environment. In the context of the control measures proposed by the applicant, and considering both the short and long term, none of these scenarios were identified as substantive risks that could be greater than negligible. The principal reasons for these conclusions are summarised in Table 3 and include: limits on the size, locations and duration of the release proposed by CSIRO controls proposed by CSIRO to restrict the spread and persistence of the GM safflower plants and their genetic material the genetic modifications are unlikely to give rise to adverse effects on human health and safety or the environment Chapter 2 – Risk assessment 24 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator widespread presence of the same and similar genes in the environment and lack of evidence of harm from them limited ability and opportunity for the GM safflower plants to transfer the introduced genetic material to commercial safflower crops or wild safflower populations none of the GM plant material or products will enter human food or animal feed supply chains. The Risk Analysis Framework (OGTR 2013), which guides the risk assessment and risk management process, defines negligible risks as insubstantial with no present need to invoke actions for their mitigation. Therefore, no additional controls are required to treat these negligible risks. Hence, the Regulator considers that the dealings involved in this proposed release do not pose a significant risk to either people or the environment. Chapter 2 – Risk assessment 25 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Chapter 3 Office of the Gene Technology Regulator Risk management plan Section 1 Background Risk management is used to protect the health and safety of people and to protect the environment by controlling or mitigating risk. The risk management plan addresses risks evaluated as requiring treatment and considers controls and limits proposed by the applicant, as well as general risk management measures. The risk management plan informs the Regulator’s decisionmaking process and is given effect through licence conditions. Under section 56 of the Act, the Regulator must not issue a licence unless satisfied that any risks posed by the dealings proposed to be authorised by the licence are able to be managed in a way that protects the health and safety of people and the environment. All licences are subject to three conditions prescribed in the Act. Section 63 of the Act requires that each licence holder inform relevant people of their obligations under the licence. The other statutory conditions allow the Regulator to maintain oversight of licensed dealings: section 64 requires the licence holder to provide access to premises to OGTR inspectors and section 65 requires the licence holder to report any information about risks or unintended effects of the dealing to the Regulator on becoming aware of them. Matters related to the ongoing suitability of the licence holder are also required to be reported to the Regulator. The licence is also subject to any conditions imposed by the Regulator. Examples of the matters to which conditions may relate are listed in section 62 of the Act. Licence conditions can be imposed to limit and control the scope of the dealings. In addition, the Regulator has extensive powers to monitor compliance with licence conditions under section 152 of the Act. Section 2 Risk treatment measures for identified risks The risk assessment of risk scenarios listed in Chapter 2 concluded that there are negligible risks to people and the environment from the proposed field trial of GM safflower. These risk scenarios were considered in the context of the scale of the proposed release (Chapter 1, Section 3.1), the proposed containment measures (Chapter 1, Section 3.2), and the receiving environment (Chapter 1, Section 6), and considering both the short and the long term. The risk evaluation concluded that no additional controls are required to treat these negligible risks. Section 3 General risk management The limits and controls proposed in the application were important in establishing the context for the risk assessment and in reaching the conclusion that the risks posed to people and the environment are negligible. Therefore, to maintain the risk context, licence conditions have been imposed to limit the release to the proposed size, locations and duration, and to restrict the spread and persistence of the GMOs and their genetic material in the environment. The conditions are detailed in the licence and summarised in this Chapter. 3.1 Licence conditions to limit and control the release 3.1.1 Consideration of limits and controls proposed by CSIRO Sections 3.1 and 3.2 of Chapter 1 provide details of the limits and controls proposed by CSIRO in their application. These are discussed in the three risk scenarios characterised for the proposed release in Chapter 2. Many of these proposed control measures are considered standard for GM crop trials and have been imposed by the Regulator in previous DIR licences. The appropriateness of these controls is considered further below. The duration of the field trial would be confined to four growing seasons, with a maximum of 10 trial sites of 5 ha during the first growing season, 15 trial sites of 10 ha during each of the second Chapter 3 – Risk management 26 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator and third seasons, and 20 trial sites of 25 ha each during the fourth season. The size and duration of the trial would limit the potential exposure of humans, vertebrates and other organisms to the GMOs (Risk Scenario 1). Only authorised personnel with appropriate training would be permitted to deal with the GMOs. A standard licence condition requires all people dealing with the GMOs to be informed of relevant licence conditions. These measures would limit the potential exposure of humans to the GMOs (Risk Scenario 1). The applicant proposes, in line with a standard DIR licence condition, that trial sites are located at least 50 m from natural waterways to minimise the chance of viable plant material being washed away from the site (Risk Scenario 2). An additional licence condition has been imposed requiring immediate notification of any extreme weather conditions affecting the trial sites during the release. This will allow contingency measures to be taken to restrict dispersal of GM safflower outside the proposed trial sites. The applicant proposes to grow both GM safflower and non-GM safflower in the trial sites. As non-GM safflower may be mingled with or fertilised by GM safflower, a standard licence condition has been imposed requiring non-GM safflower plants grown in a trial site to be treated as if they are GMOs. At the trial sites, the applicant proposes to minimise bird access to the GMOs by covering the site with bird netting, the use of commercial bird scarers and/or planting decoy crops within the Isolation Zone. These measures would limit both exposure of wildlife to the GMOs (Risk Scenario 1) and potential dispersal of GMOs outside the trial sites (Risk Scenario 2). A licence condition requires that for the period from 14 days after commencement of flowering of the GMOs in a trial site until the site has been cleaned, the trial site must be either: covered with bird netting, which must be maintained in a state adequate to deter birds; or equipped with bird scarers that are expected to deter the main seed-eating bird species present in the vicinity of the trial site; or surrounded by a 10 m strip of non-GM plants, such as sorghum, as a decoy or sacrificial crop within the Isolation Zone, and these plants must bear seed over the same period as the safflower in the Planting Area; or use a method approved in writing by the Regulator to achieve the above outcomes; or use a combination of the above methods. The applicant proposes to monitor for the presence of rodents by trapping and to control populations by baiting if necessary. Combined with the use of a monitoring zone (below) these measures should both limit exposure of rodents to the GMOs (Risk Scenario 1) and minimise potential dispersal of GMOs outside the trial sites by rodents (Risk Scenario 2). A licence condition has been imposed that requires that for the period while GMOs are being grown and until the trial site has been cleaned, measures must be implemented to control rodents within the site. The applicant proposes to surround each trial site with a 10 m monitoring zone, which is kept free of weeds and any species related to safflower, monitored for rodents and maintained in a manner to minimise rodent activity. This would serve three purposes: to avoid attracting or harbouring rodents (Risk Scenario 2) to remove safflower plants or related species that might hybridise with GM safflower (Risk Scenario 3) to facilitate detection of GM plant material that has been dispersed during sowing or harvesting (Risk Scenario 2). Chapter 3 – Risk management 27 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Therefore, a licence condition has been included requiring a 10 m monitoring zone around each trial site be maintained in a manner that does not attract or harbour rodents. Additional licence conditions require inspection of the monitoring zone while the GMOs are growing and post-harvest for detection and destruction of volunteer safflower plants. The applicant proposes that the monitoring zone would be surrounded with an isolation zone of 190 m, or 50 m if no safflower plants or related species were observed in the previous growing season, which would be inspected every 35 days while the GMOs are flowering in a trial site, and any safflower or related species destroyed. Separation of the GM crop from isolated safflower plants by 200m (10 m monitoring zone plus 190 m isolation zone) is considered sufficient for minimising gene flow to these plants, as isolated plants are less attractive to bees than are dense populations of a particular plant species. If the isolation zone is to be reduced to 50 m, additional measures should be taken to prevent the appearance of safflower plants in the remaining area. Since safflower has very low seed dormancy, the most likely cause of safflower plants occurring in this area is through planting of a safflower crop or planting of another crop which is inadvertently contaminated with safflower seeds. The proposed isolation distances have been included as a licence condition (referred to as an inspection zone, being 190 m or 50m surrounding the monitoring zone), with an additional condition that requires any seed planted in a 190 m isolation zone to be inspected for the adventitious presence of safflower seeds before planting. Separation from other safflower crops is addressed below. As indicated above, while the GMOs are flowering, the applicant has proposed to inspect the monitoring and isolation zones every 35 days for the presence of safflower and related species. Safflower requires about 45 days to develop from its inconspicuous rosette stage to flowering, but hotter or drier environments can shorten development through the rosette and elongation/branching phases (GRDC 2010). A higher frequency of inspection is considered appropriate to deal with this uncertainty. An imposed licence condition requires that for the period from 14 days prior to the expected commencement of flowering of any GMOs in a trial site, until after all GMOs have finished flowering, the monitoring and isolation zones must be inspected every 14 days for the presence of safflower, and any plants discovered must be destroyed prior to flowering. These measures would minimise gene flow to naturalised safflower (Risk Scenario 3). Although the applicant proposed to inspect for related species in these areas while the GMOs are flowering, the available evidence does not support this measure. As discussed in Chapter 1, Section 6.3, the four related species present in Australia are unlikely to hybridise with safflower and produce fertile offspring. Thus, there is no requirement in the licence to inspect for related species. The applicant proposes to ensure that no other safflower crops are grown within 400 m of the trial sites. The safflower industry is small in Australia with on average less than 10,000 hectares grown annually (ABARES 2014), and thus a commercial safflower crop is not likely to be close to any particular trial site. As discussed in Chapter 2 long-distance outcrossing in safflower can be highly variable from site to site, but generally decreases over distance with no outcrossing observed at a distance of 300 m. Variability in outcrossing rates was thought to be due to differences in pollinator numbers and site size (AOSCA 2012; McPherson et al. 2009a). There is a possibility that GM safflower could pollinate dense populations of naturalised safflower growing in the areas between 200 m and 400 m from the trial sites, but as safflower is not a common weed, the number of wild safflower plants present in these areas is likely to be very low. However, significant numbers of wild safflower plants might be present between 200 m and 400 m from the trial sites if the plants were growing as volunteers following planting of a safflower crop in the previous year. Cross-pollination events between the GMOs and other safflower crops or dense populations of wild safflower located slightly further than 400 m from the trial sites are expected to be rare. However, there is little published quantitative information about long-distance safflower gene flow. Chapter 3 – Risk management 28 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator The international guidelines for production of basic safflower seed recommend an exclusion distance of 400 m from other safflower cultivars to produce high purity seed (OECD 2013). However, at this exclusion distance there could still be very low levels of cross-pollination that are acceptable for growers, and there is a lack of scientific studies addressing efficacy of exclusion distances for safflower. In these circumstances of uncertainty, it is considered appropriate to increase the exclusion distance by a safety factor. For a Canadian field trial of GM safflower in 2011, the Canadian Food Inspection Agency (see CFIA website) required that GM safflower plants be reproductively isolated from other safflower plants by 800 m, and from safflower seed production by 1600 m. However, the GM safflower in the Canadian trial expressed a pharmaceutical compound that could potentially have adverse effects on humans or animals if ingested, justifying a large isolation distance. In the USA, field trials of GM safflower expressing pharmaceutical compound required an even larger isolation distance of 2 miles (3.2 km) to the nearest commercial safflower crop (USDA-APHIS 2008). APHIS has processed over 25 safflower permits since 2003 and has found no significant impacts to humans or the environment (USDAAPHIS 2008). In Australia, a relatively small area is planted with safflower crops, less than 10,000 ha per annum (ABARES 2014). In Risk Scenario 3, it was noted that pollen flow might occur not only to a safflower crop, but also to safflower volunteers if these grow in abundance in the year after planting of a safflower crop. Based on these considerations, the licence requires that GM safflower must not be grown within 600 m of either a safflower crop that is not a part of the field trial, or an area that was planted in the previous 12 months to a safflower crop that was not part of the field trial. This condition would minimise gene flow to other safflower plants (Risk Scenario 3). In line with a standard licence condition, the applicant proposes to clean equipment used with the GMOs on-site before use for any other purpose. Inclusion of this measure as a licence condition would minimise dispersal of GM material by humans (Risk Scenario 2). The applicant proposes to clean the trial sites and adjacent areas after harvest by incorporating plant material into the soil. During sowing and harvesting, plant material could be scattered into the area immediately surrounding the trial, so there is potential for residual seed to be present in both the trial site and the monitoring zone. In Risk Scenario 2, it was noted that the area immediately adjacent to the planting area (the monitoring zone) would be inspected for safflower plants and any plants found would be destroyed. Thus it is unlikely safflower seed dispersed into this area during sowing would germinate and persist. In Risk Scenario 2, it was also noted that during the period between harvest and cleaning, residual seed on the soil surface would be susceptible to dispersal by animal predation, water runoff after rainfall, or strong winds. Therefore, it is appropriate to require that cleaning occurs shortly after harvest. An imposed licence condition requires that GMO planting areas and their associated monitoring zones must be cleaned by ploughing plant material into the soil within 14 days after harvest of the GMOs. The applicant proposes regular watering of the trial site post-harvest, to promote germination of residual seed. Due to the low dormancy of safflower seeds and based on results from DIR 121, adequate post-harvest soil moisture or rainfall is likely to occur to an extent sufficient to encourage germination and manage survival and persistence of viable safflower seeds in the soil (Risk Scenario 2). The requirement that GMO planting areas and their associated monitoring zones must be irrigated at least once post-harvest during the volunteer-free period has been included as a licence condition. Irrigation during this period would demonstrate the depletion of any residual GM safflower seed bank. The applicant proposes post-harvest monitoring of the trial site and any areas used to clean equipment for 24 months, destroying any volunteer safflower plants detected before flowering. The proposed frequency of inspections is monthly, or if no volunteers are found during six consecutive inspections, reduced to once per three months. As safflower has low dormancy, with buried seeds reported to have no viability beyond one year (McPherson et al. 2009b), it is considered unnecessary to inspect the trial sites for as long as two years. Licence conditions require postharvest monitoring and destruction of volunteers at least once every 35 days for at least twelve Chapter 3 – Risk management 29 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator months and until no volunteers are found for at least six months. Records must be kept of monitoring activities and findings, including number and location of volunteers, which will allow the Regulator to assess the ongoing suitability of these measures and provide additional information for future assessments. In line with a standard licence condition, the applicant proposes that all transport and storage of GM plant material would comply with the Regulator’s Guidelines for the transport, storage and disposal of GMOs, available on the OGTR website. These protocols for the handling of GMOs would minimise exposure of people and other organisms to the GMOs (Risk Scenario 1), and dispersal of GMOs into the environment (Risk Scenario 2) during transport. Transport and storage of GM plant material according to the Regulator’s Guidelines has been included as a licence condition. Experiments with the GMOs or GM plant material may be conducted in certified physical containment facilities as Notifiable Low Risk Dealings (NLRDs) in accordance with all appropriate requirements of the Gene Technology Regulations 2001, and therefore this activity is not covered in the licence. The applicant does not propose using any of the GM plant material for human or animal consumption, and the GM safflower has not been assessed for food use by FSANZ. Therefore, a condition of the licence prohibits material from the trial from being used for human food or animal feed. 3.1.2 Summary of licence conditions to be implemented to limit and control the release A number of licence conditions have been imposed to limit and control the proposed release, based on the above considerations. These include requirements to: limit the duration of the field trial to four growing seasons limit the field trial to 10 trial sites of 5 ha during the first growing season, 15 trial sites of 10 ha each during both the second and third seasons, and 20 trial sites of 25 ha each during the fourth season locate trial sites at least 50 m from natural waterways ensure that no other safflower crops are grown within 600 m of the trial sites surround each site with a 10 m monitoring zone maintained in a manner that does not attract or harbour rodents while the GM safflower is flowering, inspect the monitoring zone and an isolation zone of 190 m (or 50 m if approved by the Regulator) for safflower plants, and destroy any found use bird netting, bird scarers, decoy crops or another method approved by the Regulator to deter birds control rodents harvest GM safflower separately to other crops clean equipment prior to removal from the site destroy all plant materials not required for testing or future trials promote germination of residual seed by post-harvest tillage and irrigation monitor for at least 12 months after harvest, and until no volunteer safflower plants are detected for at least 6 consecutive months, and destroy any safflower plants before flowering transport and store GM material in accordance with the Regulator’s guidelines not allow GM plant material or products to be used for human food or animal feed. Chapter 3 – Risk management 30 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator 3.2 Other risk management considerations All DIR licences issued by the Regulator contain a number of conditions that relate to general risk management. These include conditions relating to: applicant suitability contingency plans identification of the persons or classes of persons covered by the licence reporting structures a requirement that the applicant allows access to the trial sites and other places for the purpose of monitoring or auditing. 3.2.1 Applicant suitability In making a decision whether or not to issue a licence, the Regulator must have regard to the suitability of the applicant to hold a licence. Under section 58 of the Act, matters that the Regulator must take into account include: any relevant convictions of the applicant (both individuals and the body corporate) any revocation or suspension of a relevant licence or permit held by the applicant under a law of the Commonwealth, a State or a foreign country the capacity of the applicant to meet the conditions of the licence. On the basis of information submitted by the applicant and records held by the OGTR, the Regulator considers the CSIRO suitable to hold a licence. The licence includes a requirement for the licence holder to inform the Regulator of any circumstances that would affect their suitability. In addition, any applicant organisation must have access to a properly constituted Institutional Biosafety Committee and be an accredited organisation under the Act. 3.2.2 Contingency plan CSIRO is required to submit a contingency plan to the Regulator before planting the GMOs. This plan would detail measures to be undertaken in the event of any unintended presence of the GM safflower lines outside of the permitted areas. CSIRO is also required to provide a method to the Regulator for the reliable detection of the presence of the GMOs and the introduced genetic materials in a recipient organism. This instrument is required before planting of the GMOs. 3.2.3 Identification of the persons or classes of persons covered by the licence The persons covered by the licence are the licence holder and employees, agents or contractors of the licence holder and other persons who are, or have been, engaged or otherwise authorised by the licence holder to undertake any activity in connection with the dealings authorised by the licence. Prior to growing the GMOs, CSIRO is also required to provide a list of people and organisations that will be covered by the licence, or the function or position where names are not known at the time. 3.2.4 Reporting requirements The licence obliges the licence holder to immediately report any of the following to the Regulator: any additional information regarding risks to the health and safety of people or the environment associated with the trial Chapter 3 – Risk management 31 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator any contraventions of the licence by persons covered by the licence any unintended effects of the trial. A number of written notices are also required under the licence that would assist the Regulator in designing and implementing a monitoring program for all licensed dealings. The notices would include: expected and actual dates of planting details of areas planted to the GMOs expected dates of flowering expected and actual dates of harvest and cleaning after harvest details of inspection activities. 3.2.5 Monitoring for Compliance The Act stipulates, as a condition of every licence, that a person who is authorised by the licence to deal with a GMO, and who is required to comply with a condition of the licence, must allow inspectors and other persons authorised by the Regulator to enter premises where a dealing is being undertaken for the purpose of monitoring or auditing the dealing. Post-release monitoring continues until the Regulator is satisfied that all the GMOs resulting from the authorised dealings have been removed from the release site. If monitoring activities identify changes in the risks associated with the authorised dealings, the Regulator may also vary licence conditions, or if necessary, suspend or cancel the licence. In cases of non-compliance with licence conditions, the Regulator may instigate an investigation to determine the nature and extent of non-compliance. The Act provides for criminal sanctions of large fines and/or imprisonment for failing to abide by the legislation, conditions of the licence or directions from the Regulator, especially where significant damage to health and safety of people or the environment could result. Section 4 Issues to be addressed for future releases Additional information has been identified that may be required to assess an application for a large scale or commercial release of these GM safflower lines, or to justify a reduction in containment conditions. This includes: additional molecular and biochemical characterisation of the GM safflower lines, particularly with respect to production of potential toxins or allergens additional phenotypic characterisation of the GM safflower lines, particularly with respect to traits that may contribute to weediness additional information on long distance gene flow for safflower. Section 5 Conclusions of the RARMP The risk assessment concluded that this proposed limited and controlled release of GM safflower poses negligible risks to the health and safety of people or the environment as a result of gene technology, and that these negligible risks do not require specific risk treatment measures. However, conditions have been imposed to limit the release to the proposed size, locations and duration, and to restrict the spread and persistence of the GMOs and their genetic material in the environment, as these were important considerations in establishing the context for assessing the risks. Chapter 3 – Risk management 32 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator References ABARES (2014). Australia Commodity Statistics oilseeds table 161. Australian Bureau of Agricultural and Resource Economics and Sciences, Department of Agriculture, Australia. AOSCA (2012). AOSCA Standards and Procedures for Producing Certified Safflower Seed. Association of Official Seed Certifying Agencies website. Arts, J.H.E., Mommers, C., de Heer, C. (2006). Dose-response relationships and threshold levels in skin and respiratory allergy. Critical Reviews in Toxicology 36: 219-251 Bammer, G., Smithson, M. (2008). Uncertainty and risk: Multidisciplinary perspectives., Bammer, G., Smithson, M. (eds). Earthscan London. Baykal, U., Zhang, Z. (2010). Small RNA-mediated gene silencing for plant biotechnology. Chapter 11. In: AJ Catalano, ed. Gene silencing: Theory, techniques and applications. Nova Science Publishers, Inc. New York. pp 255-269. Belide, S., Hac, L., Singh, S.P., Green, A.G., Wood, C.C. (2011). Agrobacterium-mediated transformation of safflower and the efficient recovery of transgenic plants via grafting. Plant Methods 7: 1-12 Boch, R. (1961). Honeybee activity on safflower (Carthamus tinctorius L.). Canadian Journal of Plant Science 41: 559-562 Bonaventure, G., Salas, J.J., Pollard, M.R., Ohlrogge, J.B. (2003). Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15: 1020-1033 Bradford, K.J., van Deynze, A., Gutterson, N., Parrott, W., Strauss, S.H. (2005). Regulating transgenic crops sensibly: lessons from plant breeding, biotechnology and genomics. Nature Biotechnology 23: 439-444 CFIA (2015). Plants with Novel Traits (PNTs) - Approved confined research field trials / Terms and conditions. Canadian Food Inspection Agency website. Accessed on 28 January 2015. Chengaiah, B., Rao, K.M., Kumar, K.M., Alagusundaram, M., Chetty, C.M. (2010). Medicinal importance of Natural dyes-a review. Int J PharmTech Res 2: 144-154 Claassen, C.E. (1950). Natural and Controlled Crossing in Safflower, Carthamus tinctorius L. Agronomy Journal 42: 381-384 Clark, A.J. and Brinkley, T. (2001). Risk management: for climate, agriculture and policy. Commonwealth of Australia Canberra. Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human Health, N.R.C. (2004). Unintended Effects from Breeding. Chapter 3. In: Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects. The National Academies Press pp 39-71. Compes, E., Bartolome, B., Fernandez-Nieto, M., Sastre, J., Cuesta, J. (2006). Occupational asthma from dried flowers of Carthamus tinctorius (safflower) and Achillea millefolium (yarrow). Allergy 61: 1239-1240 References 33 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Cresswell, J.E. (1999). The influence of nectar and pollen availability on pollen transfer by individual flowers of oil-seed rape (Brassica napus) when pollinated by bumblebees (Bombus lapidarius). Journal of Ecology 87: 670-677 Cresswell, J.E. (2000). A comparison of bumblebees' movements in uniform and aggregated distributions of their forage plant. Ecological Entomology 25: 19-25 Cresswell, J.E. (2010). A mechanistic model of pollinator-mediated gene flow in agricultural safflower. Basic and Applied Ecology 11: 415-421 Cresswell, J.E., Osborne, J.L., Bell, S.A. (2002). A model of pollinator-mediated gene flow between plant populations with numerical solutions for bumblebees pollinating oilseed rape. Oikos 98: 375-384 Cummings, J.L., Handley, L.W., MacBryde, B., Tupper, S.K., Werner, S.J., Byram, Z.J. (2008). Dispersal of viable row-crop seeds of commercial agriculture by farmland birds: implication for genetically modified crops. Environ Biosafety Res 7: 241-252 Dajue, L., Mündel, H.H. (1996). Safflower. Carthamus tinctorius L. Promoting the conservation and use of underutilized and neglected crops. 7. Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute, Rome. Delaney, B., Astwood, J.D., Cunny, H., Conn, R.E., Herouet-Guicheney, C., MacIntosh, S., Meyer, L.S., Privalle, L., Gao, Y., Mattsson, J., Levine, M., ILSI International Food Biotechnology Committee Task Force on Protein Safety (2008). Evaluation of protein safety in the context of agricultural biotechnology. Food and Chemical Toxicology 46: S71-S97 EPA (1996). Plant pesticide inert ingredient CP4 Enolpyruvylshikimate-3-D and the genetic material necessary for its production in all plants. Report No. 61, US Environmental Protection Agency Felsot, A.S. (2000). Insecticidal genes part 2: Human health hoopla. Agrichemical & Environmental News 168: 1-7 FSANZ (2009). Application A1018 - Food Derived from High Oleic Acid Soybean Line DP305423-1. Assessment Report. Food Standards Australia New Zealand. FSANZ (2011). Application A1049 - Food Derived from Herbicide-Tolerant, High Oleic Acid Soybean Line MON87705. Assessment Report. Food Standards Australia New Zealand. Gary, N., Witherell, P.C., Lorrenzen, K., Marston, J. (1977). The Interfield Distribution of Honey Bees Foraging on Carrots, Onions, and Safflower. Environmental Entomology 6: 637640 Glover, J. (2002). Gene flow study: Implications for the release of genetically modified crops in Australia. Bureau of Rural Sciences. GRDC (2010). Raising the bar with better safflower agronomy. Grains Research and Development Corporation ACT, Australia. Groves, R.H., Hosking, J.R., Batianoff, G.N., Cooke, D.A., Cowie, I.D., Johnson, R.W., Keighery, G.J., Lepschi, B.J., Mitchell, A.A., Moerkerk, M., Randall, R.P., Rozefelds, A.C., Walsh, N.G., Waterhouse, B.M. (2003). Weed categories for natural and agricultural ecosystem management. Bureau of Rural Sciences Canberra References 34 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Harwood, J.L. (1996). Recent advances in the biosynthesis of plant fatty acids. Biochim Biophys Acta 1301: 7-56 Haslberger, A.G. (2003). Codex guidelines for GM foods include the analysis of unintended effects. Nature Biotechnology 21: 739-741 Hayes, K.R. (2004). Ecological implications of GMOs: robust methodologies for ecological risk assessment. Best practice and current practice in ecological risk assessment for genetically modified organisms.1-73. Accessed on 11 August 2004. Herman, R.A., Ladics, G.S. (2011). Endogenous allergen upregulation: transgenic vs. tradtionally bred crops. Food and Chemical Toxicology 49: 2667-2669 Ingale, S., Shrivastava, S.K. (2011). Chemical and bio-chemical studies of new varieties of safflower (Carthamus tinctorius L.) PBNS-12 and PBNS-40 seeds. Advances in Agriculture & Botanics 3: 127 Keese, P. (2008). Risks from GMOs due to horizontal gene transfer. Environmental Biosafety Research 7: 123-149 Keese, P.K., Robold, V., Myers, R.C., Weisman, S., Smith, J. (2014). Applying a weed risk assessment approach to GM crops. Transgenic Research 23: 957-969 Kuehnl, S., Schroecksnadel, S., Temml, V., Gostner, J.M., Schennach, H., Schuster, D., Schwaiger, S., Rollinger, J.M., Fuchs, D., Stuppner, H. (2013). Lignans from Carthamus tinctorius suppress tryptophan breakdown via indoleamine 2, 3-dioxygenase. Phytomedicine 20: 1190-1195 Liang, G., Zhu, Y., Sun, B., Shao, Y., Jing, A., Wang, J., Xiao, Z. (2014). Assessing the survival of exogenous plant microRNA in mice. Food Science & Nutrition 2: 380-388 Liu, Z., Li, C., Li, M., Li, D., Liu, K. (2004). The subchronic toxicity of hydroxysafflor yellow A of 90 days repeatedly intraperitoneal injections in rats. Toxicology 203: 139-143 Mayerhofer, M., Mayerhofer, R., Topinka, D., Christianson, J., Good, A.G. (2011). Introgression potential between safflower (Carthamus tinctorius) and wild relatives of the genus Carthamus. BMC Plant Biology 11: 47 McPherson, M.A., Good, A.G., Topinka, A.K.C., Yang, R.C., McKenzie, R.H., Cathcart, R.J., Christianson, J.A., Strobeck, C., Hall, L.M. (2009a). Pollen-mediated gene flow from transgenic safflower (Carthamus tinctorius L.) intended for plant molecular farming to conventional safflower. Environmantal Biosafety Research 8: 19-32 McPherson, M.A., Yang, R.C., Good, A.G., Nielson, R.L., Hall, L.M. (2009b). Potential for seed-mediated gene flow in agroecosystems from transgenic safflower (Carthamus tinctorius L.) intended for plant molecular farming. Transgenic Research 18: 281-299 Mohseni, M., Seyedabadi, M., Azizi, E., Shaiatpanahi, S.M., Esfahani, H.M., Hamedi, M., Ostad, S.N. (2011). Study on Acute and Subchronic Toxicity, Cytotoxicity and In Vitro Developmental Toxicity of Safflower Extracts of "IL 111" and "LRV 51 51" Cultivars. Indian Journalof Pharmaceutical Sciences 8: 343-352 References 35 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Monfared, L.A. (2013). Histological, ultrastructural and biochemical studies on the kidney of mice treated with Carthamus tinctorius L. extract. Avicenna Journal of Phytomedicine 3: 272278 Mündel, H.H., Blackshaw, R.E., Byers, J.R., Huang, H.C., Johnson, D.L., Keon, R., Kubik, J., McKenzie, R., Otto, B., Roth, B., and Stanford, K. (2004). Safflower production on the Canadian prairies: revisited in 2004. Agriculture and Agri-food Canada. Nabloussi, A., Velasco, L., Fernandez-Martines, J.M. (2013). Cross pollination of safflower (Carthamus tinctorius L.) under Moroccan environmental conditions. International Journal of Plant Breeding 7: 145-147 Nobakht, M., Fattahi, M., Hoormand, M., Milanian, I., Rahbar, N., Mahmoudian, M. (2000). A study on the teratogenic and cytotoxic effects of safflower extract. Journal of Ethnopharmacology 73: 453-459 OECD (2013). Annex VII to the Decision: OECD Scheme for the Varietal Certification of Crucifer Seed and Other Oil or Fibre Species. Organisation for Economic Co-operation and Development website OGTR (2013). Risk Analysis Framework. The Office of the Gene Technology Regulator, Canberra, Australia. OGTR (2015). The Biology of Carthamus tinctorius L. (safflower). Petrick, J.S., Brower-Toland, B., Jackson, A.L., Kier, L.D. (2013). Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regulatory Toxicology and Pharmacology 66: 167176 Pheloung, P.C., Williams, P.A., Halloy, S.R. (1999). A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. Journal of Environmental Management 57: 239-251 Singh, V., Nimbkar, N. (2006). Safflower (Carthamus tinctorius L.). Chapter 6. In: Genetic Resources, Chromosome Engineering, and Crop Improvement: Oilseed Crops. CRC Press pp 167-194. Standards Australia New Zealand, CRC for Australian Weed Management (2006). HB 294:2006 National Post-Border Weed Risk Management Protocol. Standards Australia; Standards New Zealand; pp 1-68. Steiner, H.Y., Halpin, C., Jez, J.M., Kough, J., Parrott, W., Underhill, L., Weber, N., Hannah, L.C. (2013). Evaluating the potential for adverse interactions within genetically engineered breeding stacks. Plant Physiology 161: 1587-1594 USDA-APHIS (2008). Finding of no significant impact and decision notice (permit application 06-363-103r). Vanhercke, T., Wood, C.C., Stymne, S., Singh, S.P., Green, A.G. (2013). Metabolic engineering of plant oils and waxes for use as industrial feedstocks. Plant Biotechnology Journal 11: 197-210 References 36 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Velasco, L., Fischer, M., Fernandez-Martinez, J.M. (2012). Short communication. Estimation of cross-fertilization rate in safflower (Carthamus tinctorius L.). Spanish Journal of Agricultural Research 10: 155-159 Waines, J.G., Hegde, S.G. (2003). Intraspecific gene flow in bread wheat as affected by reproductive biology and pollination ecology of wheat flowers. Crop Science 43: 451-463 Weber, N., Halpin, C., Hannah, L.C., Jez, J.M., Kough, J., Parrott, W. (2012). Editor's choice: crop genome plasticity and its relevance to food and feed safety of genetically engineered breeding stacks. Plant Physiology 160: 1842-1853 Zhang, L., Hou, D., Chen, X., LI, D., Zhu, L., Zhang, Y., Li, J., Bian, Z., Liang, X., Cai, X., Yin, Y., Wang, C., Zhang, T., Zhu, D., Zhang, D., Xu, J., Chen, Q., Ba, Y., Liu, J., Wang, Q., Chen, J., Wang, J., Wang, M., Zhang, Q., Zhang, J., Zen, K., Zhang, C.Y. (2011). Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 22: 107-126 Zhang, Y., Zhang, Z., Zhu, Q., Xu, X. (2009). Experimental study of anaphylaxis caused by Carthamus tinctorious (Safflower) injection in guinea pig model. The Chinese Journal of Clinical Pharmacology 6: 514-517 Zhou, X., Tang, L., Xu, Y., Zhou, G., Wang, Z. (2014). Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. Journal of Ethnopharmacology 151: 27-43 References 37 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Appendix A Summary of submissions from prescribed experts, agencies and authorities6 Advice received by the Regulator from prescribed experts, agencies and authorities on the consultation RARMP is summarised below. All issues raised in submissions that related to risks to the health and safety of people and the environment were considered in the context of the currently available scientific evidence and were used in finalising the RARMP that formed the basis of the Regulator’s decision to issue the licence. Abbreviations:; GM: Genetically Modified; RARMP: Risk Assessment and Risk Management Plan. Sub.No: Summary of issues raised Comment 1 Council is grateful for opportunity but notes the lack of scientific expertise to comment on the RARMP. Noted 2 Notes Council will not be submitting comments on this application. Noted 3 Noted that the RARMP was circulated for comment and no adverse comments were received. Indicated no objection to the issue of a licence for DIR 131. Noted 4 Noted no evident difficulties or problems Noted associated with this release, particularly given the small scale of production. 5 Noted agreement with the RARMP that Noted this release poses negligible risks to human health and safety or to the environment where conducted under the proposed limits and controls. 6 Supported the conclusions of the RARMP that the proposed dealings posed negligible risks to human health and safety or to the environment. Noted 7 Noted that given the control measures, the limited scale and duration of the release, the environmental risks posed by the trial of the GM safflower lines are likely to be low and manageable. Noted Noted that according to McPherson et al (2004) safflower is one of the most serious, difficult and costly thistle weeds in New South Wales. McPherson et al. (2004) is referring to Carhamus lanatus as a serious weed, whereas safflower (Carthamus tinctorius) the subject of the proposed release, is considered a minor weed that primarily establishes on disturbed ground (Chapter 1, section 6.3) 6 Prescribed agencies include GTTAC, State and Territory Governments, relevant local governments, Australian Government agencies and the Minister for the Environment. Appendix A 38 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Sub.No: 8 9 Appendix A Office of the Gene Technology Regulator Summary of issues raised Comment Indicated that the RARMP would be improved if it addressed possible hazards from traits that could provide competitive advantage to wild safflower and related species. The GM safflower is highly unlikely to successfully cross pollinate with any other species under natural conditions. Crosses made under artificial conditions tend to be sterile and often require intervention such as embryo rescue and treatment with colchicine. The introduced traits (increased oleic acid content in the seed & selectable markers) are not known to enhance weediness in plants. The licence conditions imposed will minimise outcrossing and persistence of any offspring (Chapter 1, section 6.3 and Chapter 2, sections 2.4.2 & 2.4.3). Indicated phenotypic changes in GM Arabidopsis plants modified to disrupt the FATB gene (see Bonaventure et al. (2003)). Expression of FATB gene is also modified in the GM safflower plants. In light of the Bonaventure paper, it is considered the RARMP is lacking in specific details on how and what phenotypic data was collected on the GM safflower plants. The Bonaventure article examined disruption of FATB expression in Arabidopsis. The phenotypic changes in the GM Arabidopsis noted in this article included: ~50% reduction in plant size & fresh weight at the four-week stage, delayed flowering by 2 weeks, shorter stems and ~50% reduction in seed germination. Approximately 20% of the seed were very deformed and had a germination rate of 16%. It is unlikely that any of the above changes could lead to an increase in weediness of the GM Arabidopsis plants compared to the nonGM parental plants. The applicant has indicated that the GM safflower lines proposed for release have been examined over several generations, some lines up to the 5th generation. The GM lines have been grown in glasshouses and/or in field trials under DIR121. Observations on these plants indicate no phenotypic differences between the GM and non-GM safflower for germination rate, seed count per plant, time to flowering, plant height and fertility (Chapter 1, section 5.4, Chapter 2, section 2.4.2). Such gross phenotypic changes observed in the GM Arabidopsis would be difficult to overlook if they had occurred in the GM safflower. The proposed trial will provide further phenotypic data on the GM safflower lines under a variety of Australian agricultural environments. Agrees with the identified uncertainty and thus has indicated a desire for more specific guidance on future data requirement, especially on traits related to increased weediness such as: relative growth rates, including early vigour effect of introduced traits on grain size, altered root structure and root depth seed dormancy and germination disease resistance other characteristics of weediness such as shattering, seed dispersal or seed longevity. Is supportive of the application as the consultation RARMP indicates that the proposed release poses negligible risks to people or the environment. The RARMP indicates areas of uncertainty and issues to be addressed for future releases (Chapter 2, sections 3 & 4, Chapter 3, Section 4). The issues raised by the submitter would be addressed under the second dot point in Chapter 3, section 4: additional phenotypic characterisation of the GM safflower lines, particularly with respect to traits that may contribute to weediness Rather than specifying particular experiments or data requirements, RARMPs provide broad guidance on areas of uncertainty to be addressed in possible future applications larger scale or commercial release. However, the OGTR does routinely discuss specific information requirements with applicants prior to submission of such an application. Agrees with the overall conclusions of the RARMP Noted Noted 39 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Sub.No: Summary of issues raised Comment The Regulator should consider whether the proposed limits are appropriate for this trial and whether larger trial sites can be adequately managed The size and number of trial sites, as well as the proposed limits and controls all formed part of the risk context (Chapter 1) and were taken into account when assessing risks (Chapter 2) and the appropriateness of these measures in managing any identified risks (Chapter 3). The risk assessment concluded that this proposed limited and controlled release of GM safflower poses negligible risks to the health and safety of people or the environment as a result of gene technology, and that these negligible risks do not require specific risk treatment measures. However, conditions have been imposed to limit the release to the proposed size, locations and duration, and to restrict the spread and persistence of the GMOs and their genetic material in the environment, as these were important considerations in establishing the context for assessing the risks. Noted, inconsistencies corrected as appropriate. Some minor inconsistencies in the RARMP were noted. Appendix A Office of the Gene Technology Regulator 40 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator Appendix B Summary of submissions from the public The Regulator received 3 submissions from the public on the consultation RARMP. The issues raised in these submissions are summarised in the table below. All issues raised in the submissions that related to risks to the health and safety of people and the environment were considered in the context of currently available scientific evidence in finalising the RARMP that formed the basis of the Regulator’s decision to issue the licence. Issues raised: C: containment; H: Health; M: Marketing. Other abbreviations: FSANZ: Food Standards Australia New Zealand; GM: Genetically modified; RARMP: risk assessment and risk management plan. Sub. Issue No: 1 2&3 Summary of issues raised Comment C Objects to the number of trial sites with no proof of the ability of the applicant to decontaminate (clean) the sites. Licence conditions allow field trials of the GM safflower at up to a total of 60 sites on a maximum cumulative area of 850 ha over a 4 year period. However, the number of sites allowed per year and the size of the sites rises over the four year period from 5 sites of a maximum of 10 ha each, to 20 sites of 25 ha each in the final year. Thus, as some sites will meet post-harvest requirements and be signed off each year, it is unlikely that the applicant will deal with 60 sites at one time. Licence conditions for DIR 131 require the licence holder to provide the Regulator with inspection reports on the trials as they progress and during the postharvest monitoring phase. Additionally, OGTR inspectors will visit trial sites to ensure the licence holder is compliant with licence conditions. The sites will only be signed off once the Gene Technology Regulator (the Regulator) is satisfied that no GM safflower material remains. H The high oleic levels may have negative impacts for human health. As this application is for a limited and controlled release (field trial), a licence condition has been imposed to prohibit GM plant material or products being used for human food or animal feed. Currently non-GM high oleic acid (70-80%) safflower cultivars are grown in Australia. Oils high in oleic acid are used as cooking oils and in cosmetics and infant food formulations. FSANZ is responsible for human food safety assessment and would have to approve the use of GM safflower or products derived from it prior to their use for human food. C Does not see the point of ‘restricting’ the spread and persistence of the GMOs because if any of the GMOs were uncontained their populations and cross-populations may increase at any time regardless of how many were released. Strict licence conditions have been imposed to limit the area and duration of the trial, as well as to restrict the spread and persistence of the GMOs beyond the trial. These include obligations on the licence holder to monitor for and destroy any GMOs remaining in the environment following the trial. The genetic modifications are unlikely to increase the weediness of the GM safflower plants as the genetic modifications provide no known selective advantage compared to non-GM safflower. - Object to trials of GM safflower oil unless they are in a securely confined place. Suggest rejecting DIR 131. Noted. Appendix B 41 DIR 131 – Risk Assessment and Risk Management Plan (February 2015) Office of the Gene Technology Regulator H Health safety studies have not been done, so these GM organisms should not be released to the open-air environment until animal feeding studies and human clinical trials have been done which would test for toxicity and allergenicity responses. As this application is for a limited and controlled release (field trial), a licence conditions has been imposed to prohibit GM plant material or products being used for human food or animal feed. FSANZ is responsible for human food safety assessment and would have to approve the use of GM safflower or products derived from it prior to their use for human food. H Fish and seafood are well-known triggers for allergic, even anaphylactic reactions. To force jellyfish genes into the safflower DNA may cause catastrophic side-effects in some people when ingested, inhaled or absorbed through the skin. The gfp gene (derived from jellyfish) and its products have been considered in detail in previous RARMPs (for example DIR 096/2009) and assessed as posing negligible risk to human or animal health or to the environment by the Regulator. As indicated in Chapter 2, section 2.1 of the RARMP, further information about this gene can be found in the document ‘Marker genes in GM plants’ available from the Risk Assessment References page on the OGTR website. See also response to issue above. C There is a history of pollen and GMOs escaping from GMO trials, which OGTR must consider Strict licence conditions have been imposed to restrict the spread of the GMOs and their genetic material. These include conditions to isolate trial sites from other safflower plants, cleaning of equipment used with GM plants, secure transport and storage of GM plant material, and obligations on the licence holder to monitor for and destroy any GMOs remaining in the environment following the trial. M Questioned the demand for GM safflower that contains animal or bacterial genes and stated that Australians do not want to consume anything transgenic (submitter 2 only). The Act requires the Regulator to identify and manage risks to human health and safety and the environment posed by or as a result of gene technology. Matters related to marketing or consumer preferences are outside the Regulator’s legislative responsibility. These matters are addressed by the States and Territories and industry. Appendix B 42