Haemolytic disease of the fetus and newborn (HDFN)
advertisement

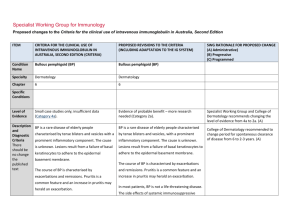
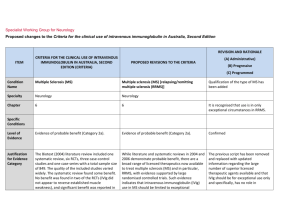
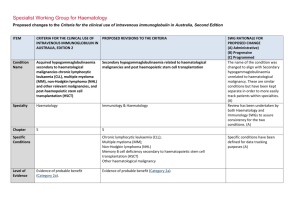
Specialist Working Group for Haematology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia ITEM Condition Name Condition Short Name CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED V3 CHANGES TO THE CRITERIA AND BLOODSTAR RATIONALE FOR PROPOSED V3 CHANGES Haemolytic disease of the newborn (HDN) HDN Haemolytic disease of the fetus and newborn (HDFN) HDFN Change of name to include recognition of the fetus. Haematology Haematology 7 7 No change. Small case studies only; insufficient data (Category 4a) Small case studies only; insufficient data (Category 4a) No change. HDN arises from foetomaternal antigen incompatibility and can HDN arises from fetomaternal antigen incompatibility and can result in clinically No change Used in drop down lists or where space is limited Specialty Keywords Used for key word search. Separate with semi colon Chapter Specific Conditions List all specific conditions separated by semi-colon Level of Evidence There should be no change to the published text Description and Diagnostic ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) There should be no change to the published text result in clinically significant foetal/neonatal haemolysis, severe anaemia and hyperbilirubinaemia. significant foetal/neonatal haemolysis, severe anaemia and hyperbilirubinaemia. Justification for Evidence Category Although prophylaxis programs have reduced the frequency of Rhesus (Rh) D HDN, antibodies to RhD remain the most common cause in Australia. Antibodies to other antigens in the Rh system (e.g. Rhc, E), ABO and other antigens (e.g. K) may also cause disease ranging from mild to lifethreatening. Although prophylaxis programs have reduced the frequency of Rhesus (Rh) D HDN, antibodies to RhD remain the most common cause in Australia. Antibodies to other antigens in the Rh system (e.g. Rhc, E), ABO and other antigens (e.g. K) may also cause disease ranging from mild to life threatening. There should be no change to the published text Diagnosis is required Diagnosis must be verified Exclusion Criteria PROPOSED V3 CHANGES TO THE CRITERIA AND BLOODSTAR RATIONALE FOR PROPOSED V3 CHANGES SWG have suggested updating with the addition of recommendation 7 from the Patient Blood management guideline – module 7 – paediatric and neonatal. In infants with HDFN, the routine use of IVIg is not recommended in national Australian clinical guidelines. By which speciality By which speciality Bullet list of exclusion criteria Indications Qualifying Criteria IVIg may be used in selected cases in consultation with experts in foetomaternal medicine and National Blood Authority IVIg may be used in selected cases in consultation with experts in fetomaternal medicine and transfusion medicine. pg. 2 Spelling correction to fetomaternal. Addition of Expert opinion ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) transfusion medicine. Review Criteria Dose Refer to the current product information sheet for further information. The aim should be to use the lowest dose possible that achieves National Blood Authority PROPOSED V3 CHANGES TO THE CRITERIA AND BLOODSTAR In maternity patients with a fetus affected by HDFN who is at high risk of early fetal hydrops or death, a course of weekly IVIg should be considered. (Expert opinion point 4, Patient Blood Management Guidelines – Module 6 – Paediatric and neonatal, Section 3.5.2.) RATIONALE FOR PROPOSED V3 CHANGES point 4 from the recent drafting of the Patient Blood Management Guidelines Module 6 - paediatric and neonatal. Further consideration to formal criteria and dose will be given to this condition when chapter 7 is re-developed in the next phase of the SWG work program. Refer to the current product information sheet for No change. further information. The aim should be to use the lowest dose possible that achieves the appropriate clinical outcome for each patient. pg. 3 ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED V3 CHANGES TO THE CRITERIA AND BLOODSTAR RATIONALE FOR PROPOSED V3 CHANGES the appropriate clinical outcome for each patient. BIBLIOGRAPHY Gottstein, R & Cooke, RW 2003, ‘Systematic review of intravenous immunoglobulin in haemolytic disease of the newborn’, Archives of Disease in Childhood – Fetal Neonatal Edition, vol. 88, no. 1, pp. F6–10. Kaplan, M, Vreman, HJ, Hammerman, C, et al 1996, ‘Intravenous immune globulin in neonatal ABO isoimmunisation: factors associated with clinical efficacy’, Biology of the Neonate, vol. 70, pp. 69–72. Miqdad, AM, Abdelbasit, OB, Shaheed, MM, et al 2004, ‘Intravenous immunoglobulin G therapy for significant hyperbilirubinaemia in ABO haemolytic disease of the newborn’, Journal of Maternal-Fetal and Neonatal Medicine, vol. 16, no. 3, pp. 163–6. Patient Blood Management guidelines – Module 6 – Paediatric and neonatal, section 3.5.2 END OF DOCUMENT National Blood Authority pg. 4 National Blood Authority pg. 5