Sjön Therapeutics: Executive Summary & Business Plan
advertisement

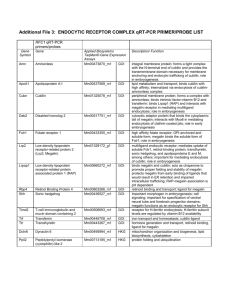
Sjön Therapeutics: Executive Summary & Business Plan Executive Summary: Sjön Therapeutics (http://www.sjontherapeutics.com) is envisioned as a global biotechnology venture founded by Mr. Somesh R. Doddi, a nobel prize in medicine candidate (http://www.doddisnobelnomination.blogspot.com) on the same KEY technology pillars as his nobel research work. Accordingly, the firm valuation (using nobel prize in medicine award proceeds as a benchmark) is at USA $1,700,000. The KEY technology pillars, through ‘technology integration’ deliver KEY intellectual property formulations using adeno-associated virus (aav) to treat via human gene therapy the conditions of cancer (lung, liver), HIV/AIDS (integrase inhibitor), insulin resistant diabetes, and 'telomere extension' to slow human aging. The entire aav product portfolio offering consists of approximately 20 unique products from one technology platform- With the average product life cycle per product of 2 years, the cumulative company’s pipeline is projected to be 40 years. This is the hallmark of a sustainable enterprise. The global gene therapy industry is expected to reach USA $325 million by the end of 2015 (see attached .pdf report). The market structure is stratified, and the current conditions are ‘hot’ for entry of a large-sized market player into the field of human gene therapy. Lonza is a prime large-sized ‘first market entrant’ (see attached .pdf report). The conclusion is that after human genome sequencing becomes commonplace and an accepted part of standard clinical/medical practice, human gene therapy will grow by leaps and bounds. AAV technology has gained respect in research and clinical trials, and will be here to stay for the beneficial future of mankind. This will make Sjön Therapeutics a key player in the future of healthcare, mankind, and society. Prepared exclusively by Mr. Somesh R. Doddi, Founder, Sjön Therapeutics Page 1 Business Plan: Valuation The intellectual property is a direct byproduct of my work for the nobel prize in medicine (http://www.doddinobelnomination.blospot.com) in KEY disease areas (cancer, HIV/AIDS, insulin resistant diabetes, aging) so the KEY intellectual property is valued at the winning amount of the nobel prize in medicine proceeds viz, USD $1,200,000. In addition, the same technology platform can yield ~≥15 more unique products for human conditions and this intellectual property is valued at conservative USD $500,000. The total firm value and selling price is ~USD $1,700,000. At present, I am offering a one-time (upto 30%) ownership opportunity via a private equity offering of 10,000 shares valued at USD $50/share. Product Portfolio The intellectual property consists of the KEY human diseases/conditions of cancer (lung, liver), HIV/AIDS, insulin resistant diabetes, and 'telomere extension' to slow human aging. This is not the end of the story though; because the potential product portfolio and growth is diverse and enormous as outlined below: Microgram Portfolio Human conditions: Leber’s Congenital Amaurosis (eye), goi: RPE65 gene Wet form of age related macular degeneration (eye) (brand name:, company: Avalanche Biotechnologies), goi: PEDF Dry form of age related macular degeneration (eye) (brand name: RST-001, company: Retrosense Therapeutics), goi: CD59 Retinitis Pigmentosa (eye) (brand name: RST-001, company: Retrosense Therapeutics), goi: RHO gene X-linked retinoschisis (eye), goi: RS-1 gene Select Clinical Trials of Gene Therapy for Retinal Diseases CLINICAL STUDY RETINAL DISEASE NAME/VECTOR MECHANISM OF ACTION SPONSOR/INSTITUTION AMD (EXUDATIVE) AAV2-SFLT01 EXPRESSES ANTI-VEGF PROTEIN GENZYME/SANOFI PHASE 1 CHORIODEMIA rAAV2.REP1 ENCODES Rab-ESCORT PROTEIN IMPERIAL COLLEGE OF LONDON PHASE 1 (REP-1) OXFORD UNIV/MOORFIELDS PRODUCES RPE65 GENE CHILDREN'S HOSPITAL OF LEBER'S CONGENITAL AAV2-hRPE65v2 AMAUROSIS RETINITIS PIGMENTOSA (AR) PHASE PHASE III PHILADELPHIA rAAV2-VMD2- PRODUCES MERTK KING KHALED EYE HOSPITAL PHASE I EXPRESSES ABC4 GENE OXFORD BIOSCEINCES/ PHASE I hMERTK STARGARDT'S STARGEN SANOFI USHER'S SYNDROME (1B) USHSTAT PRODUCES MY07A PROTEIN OXFORD BIOMEDICA Prepared exclusively by Mr. Somesh R. Doddi, Founder, Sjön Therapeutics PHASE I Page 2 Milligram/Viral gm Portfolio (human clinical trial) Human conditions: Beta thalassemia, goi: Beta-globin gene Glioma, goi: herpes simplex virus thymidine kinase (HSV-tk) Chronic heart failure, goi: FGF-4, FGF-5, VEGF Cystic Fibrosis, goi: CFTR cDNA (4450 bp) Hemophilia (B), goi: coagulant factor IX Canavan’s disease, goi: ASPA gene Parkinson’s disease, goi: tyrosine hydroxylase (th), L-amino acid decarboxylase (aadc), GDNF gene Lung Cancer (quiescence), goi: p130/RBL2, rep: 2, cap: 5. Lung Cancer (apoptosis), goi: E1-A adenoviral protein w/radioactive thymidine, rep: 2, cap: 5. Liver Cancer (quiescence), goi: p130/RBL2, rep: 2, cap: 3. Liver Cancer (apoptosis), goi: E1-A adenoviral protein w/radioactive thymidine, rep: 2, cap: 3. HIV/AIDS, goi: 2,5-diketo-D-gluconic acid reductase A, rep: 2, cap: 2. Insulin resistant (type 2) diabetes, goi: Acrp30, rep: 2, cap: 2. Slow aging, goi: hTERT, rep: 2, cap: 9. Duchenne’s muscular dystrophy, goi: minidystrophin genes Pancreatitis (brand name: Glybera, company: Uniqure), goi: LPL gene Market Analysis The current state of affairs is that there are small, mid-size, and large players in the industry. The small players due to their restricted funding and capital structure have decided to focus on production of small, purified aav virus for application in a variety of human eye conditions/disease. The production process is inexpensive although labor intensive, but small quantities of product can be sold at premium prices. The mid-size players have a ‘fill and finish’ attitude wherein they will produce the product on ‘fee for service’ basis. Pay them, and they will produce the product. The large players have currently not gotten into the human gene therapy market, with the exception of Lonza. By large players, I am referring to companies the size of Novartis and Sanofi-Aventis. Prepared exclusively by Mr. Somesh R. Doddi, Founder, Sjön Therapeutics Page 3 Strategy With adequate capital funding, manufacturing and purification can be performed in-house. Alternatively, the company intends to outsource it's manufacturing component through joint venture/partnership agreements in which the external company will finance the manufacturing of the products. So far there is one potential Swiss company that might buy my proposition; all others have indicated they will manufacture on 'a fee for service' basis. The future management may have to invest significant due diligence in seeking suitable joint venture candidates/partners. Mr. Somesh R. Doddi has attempted to seek entry into a key strategic alliance which will serve as the industry benchmark: The Virovek/Avalanche Biotechnologies/Lonza adeno-associated virus (aav) deal in which Virovek will license it’s aav production technology to Avalanche Biotechnologies, and an agreement will be signed between Avalanche Biotechnologies and Lonza. Deliverables Upon completion of sale, the following deliverables will be assigned and delivered to the client. Webmaster and web-editing access (with password) to http://www.sjontherapeutics.com Technical notes: Small scale (ug) production of virus using roller bottle technology. Technical notes: Large scale (mg/vg) production of virus using bioreactor. Raw materials: An itemised list of vendors for purchase/sourcing of raw materials. Financials INCOME STATEMENT: JAN 1, 2012- DEC 31,2012 Debits Credits Revenues 23andMe DNA spit kit Proprietary product pipeline Human genome service(s) Research products Expenses Corporate website hosting (annual) 0 0 0 0 22 Net Income -22 BALANCE SHEET: DEC 31,2012 Debits Assets Securities Issued based on valuation Credits Liabilities 0 17,00,000 Owner's Equity 17,00,000 NOTE: All currency is in USA dollars. Future Engagement: Mr. Somesh R. Doddi has expressed interest in being an ongoing Board Member of Sjön Therapeutics; handling managerial or scientific advisory responsibilities. In return for his service, he asks for an annual and recurring compensation package equivalent to: USD $30,000 + stock ownership (private or public) in Sjön Therapeutics. Prepared exclusively by Mr. Somesh R. Doddi, Founder, Sjön Therapeutics Page 4