Supplemental Information Hippocampal and Insular Response to
advertisement

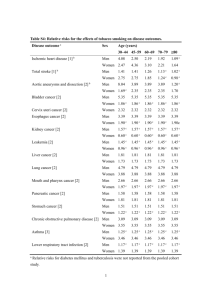
Supplemental Information Hippocampal and Insular Response to Smoking-Related Environments: Neuroimaging Evidence for Drug Context Effects in Nicotine Dependence F. Joseph McClernon, Cynthia A. Conklin, Rachel V. Kozink, R. Alison Adcock, Maggie M. Sweitzer, Merideth A. Addicott, Yinghui Chou, Nan-kuei Chen, Matthew B. Hallyburton, Anthony M. DeVito Appendix: Supplemental Methods Table S1: Demographics Table S2: Whole Brain Analyses Results Figure S1: Examples of Personal Smoking and Nonsmoking Environments Figure S2: In-scanner Craving Scores Figure S3: ROI Locations and Results Table S3: ROI Effect Sizes Figure S4: ICA Components Supplemental References 1 Appendix: Supplemental Methods Additional information regarding the acquisition and nature of personal environment cues. Using methods validated in a previous study (1) each participant was interviewed to determine four to five specific environments in which they frequently smoke (i.e. smoke at least 7 out of 10 times they are there) or abstain (smoke less than 3 out of 10 times there) and then were trained to acquire images using a digital camera. As in previous studies, four pictures of each environment (2 approaching the environment, 2 from within the environment) were acquired. Upon returning the camera an experimenter reviewed and edited, as necessary, each picture to remove proximal smoking cues (e.g. ashtrays, packs of cigarettes) and any people. Participants took between 7 to 21 days to complete the picture-taking phase. Additional information on scanning parameters. Imaging was conducted using a 3T General Electric MR750 scanner equipped with 50 mT/m gradients. Prior to imaging, the anterior and posterior commissures were identified in the midsagittal slice of a localizer series. Blood oxygen level dependent (BOLD) imaging were acquired using gradient-recalled inward spiral pulse imaging with Kspace trajectory (34 slices, TR = 1500 ms, TE = 30 ms, FOV = 24.0 cm, matrix = 64x64, flip angle = 60°, slice thickness = 4 mm, resulting in 3.75x3.75x4 mm voxels). This sequence increases signal to noise ratio and reduces dropout artifacts at the tissue-air interface (2). High resolution anatomical imaging was conducted at the end of the fMRI session using a high resolution 3-dimensional fast spoiled gradient recalled echo anatomical sequence with a 1 mm isotropic voxel size. Additional information on participant eligibility. A total of 67 individuals were screened for the study, of which 50 met eligibility criteria; 10 individuals were either withdrawn, dropped out, or lost to contact prior to the scanning session. At screening, serious health problems were assessed by a self-report questionnaire which included items asking about illnesses, operations, hospitalizations, serious injuries/accidents, heritable diseases, and medications. Additional information on data preprocessing, quality control and censoring. Nine subjects were excluded from imaging analysis due to excessive motion (i.e. motion in any direction exceeded voxel dimension length). In addition, one subject was excluded for excessive drowsiness during presentation of cue-reactivity task while in the scanner. Two participants were excluded from the analyses of lab based measures due to noncompliance during the cue-exposure session (i.e. spoke on cell phone during task; n=2) and one participant dropped from the study prior to completing the cue-exposure sessions (n=1). Additional information on outlier detection. Prior to conducting the correlational analyses, data were evaluated for outliers using studentized residuals and Cook’s D values. In addition, a robust regression with M estimation was run to identify outliers. Within the data set used for the correlation analyses, one individual was identified as an outlier consistently across all outlier detection methods and was excluded from the analysis. Additional information on Independent Component Analysis. We used the probabilistic independent component analysis (PICA) with multi-session temporal concatenation approach, implemented in MELODIC tool of the FSL (www.fmrib.ox.ac.uk/fsl), to decompose task-related, multiple 4D data sets into 20 independent components (IC) (Beckmann and Smith, 2004). Data preprocessing consisted of 1) motion correction, 2) slice time correction, 3) removal of non-brain structures, 4) spatial smoothing (5 mm FWHM Gaussian kernel), 5) registration to the MNI 152 standard space, 6) resampling data to 4 mm isotropic resolution, 7) regressing out time course profiles of the white matter and cerebrospinal fluid regions, and 8) high-pass filtering with a frequency cutoff at 150 s. In inspecting the maps associated with each of the 20 components, we identified one—IC 7—that overlapped both with our bilateral insula (centered on +/-38,10,6) and posterior hippocampus ROIs (see Figure S4). We then used FSL’s dual regression to calculate the subject specific orthogonal timecourses and spatial maps for each independent component (IC) (Beckmann et al., 2009). We then extracted the average connectivity values from the central voxel for each of the four ROIs for each individual for IC 7. The extracted values, expressed as a Fisher’s z transformed r-value, represent the connectivity between each ROI and IC 7 for each participant. The relationship between these values and smoking behavior were then assessed with Pearson correlation analysis (see Figure 5). Reference: Beckmann, C. F., Mackay, C. E., Filippini, N., and Smith, S. M. (2009). Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage 47, S148. Table S1. Demographics. Demographic information is displayed for the full sample, the sample of participants used in the analyses of behavioral data, and the sample of participants who provided useable imaging data. 2 Full Sample (n=40) Age Cigarettes/Day FTND Female Race White Black Asian Other Behavior SubSample (n=37) 23 (59.0) Mean (SD) 35.5 (11.3) 14.9 (6.6) 4.4 (1.9) n (%) 23 (62.1) 14 (35.0) 20 (50.0) 5 (12.5) 1(2.5) 13 (35.1) 18 (48.6) 5 (13.5) 1(2.7) 35.0 (11.1) 14.8 (6.5) 4.2 (2.0) fMRI SubSample (n=30) 35.4 (11.5) 14.9 (6.7) 4.1 (1.9) 17 (56.7) 13 (43.3) 12 (40.0) 5 (16.7) 0 (0.0) 3 Table S2. Whole Brain Analyses Results. Brain regions identified for each of the planned whole-brain analyses: 1) Personal Smoking Environments > Personal Nonsmoking Environments (PSE>PNE) 2) Personal Smoking Environments > Standard Smoking Environments, relative to nonsmoking environments (Personal > Standard), and 3) Smoking Environments > Proximal Smoking Cues, relative to nonsmoking environments and cues (Environment > Proximal). Cluster size, brain region, Z-value and coordinates are provided for each peak voxel. S>N = Smoking > Nonsmoking. Cluster Size Hemisphere Region Z Personal Smoking Environments > Personal Nonsmoking Environments 17764 Left Superior Parietal L. Left Precuneous Right SMA Right Posterior Cingulate G. 1423 Right Cerebellum 860 Right Planum Temporale Right Supramarginal G. Right Superior Temporal G. Right Middle Temporal G. 541 Right Frontal Operculum/Insula Right Insula Right WM/Putamen Right Caudate Right Insula 445 Right Supramarginal G. 441 Left Cerebellum x y z 4.87 4.77 4.68 4.58 4.32 4.15 3.96 3.94 3.75 3.97 3.6 3.58 3.54 3.54 4.22 3.85 -8 -10 4 14 24 56 56 62 48 34 42 26 16 36 64 -8 -54 -70 -6 -34 -50 -30 -40 -28 -28 16 8 10 12 8 -24 -38 72 46 68 44 -50 16 6 10 -4 10 -4 12 20 -4 44 -44 Personal (Smoking >Nonsmoking) > Standard (Smoking>Nonsmoking) 565 Left Putamen Left Insula Left WM 3.84 3.82 3.25 -30 -36 -22 -4 -2 0 10 -4 24 Environment (Smoking>Nonsmoking) > Proximal (Smoking >Nonsmoking) 2641 Right Lateral Occipital C. 1551 Left Lateral Occipital C. 482 Right Planum Temporale Right WM 434 Right Lateral Occipital C. Right Superior Parietal L. Right Precuneous 5 5.11 4.48 3.24 4.33 3.81 3.67 40 -40 56 42 18 10 10 -64 -68 -30 -26 -56 -50 -56 10 8 14 -4 72 72 50 4 Figure S1. Examples of Personal Smoking and Nonsmoking Environments. Stimuli employed in the study consisted of smoking and nonsmoking proximal, standard environment and personal environment cues. Proximal cues depicted smoking (e.g. cigarette in an ashtray) and nonsmoking (e.g. pencils, notepads) objects. Standard environment cues were those developed and validated in prior research (1, 3) and depicted settings associated with either a high (e.g. bus stop) or low probability (e.g. school) of smoking. These environments were devoid of proximal smoking cues and people and were shown in previous studies to result in robust cue-provoked craving (1, 3). All participants viewed the same set of standard environment cues. Examples of personal smoking and nonsmoking environment cues acquired by each participant using the protocol described above are shown. Nonsmoking Smoking Example Personal Environment Cues 5 Figure S2: In-scanner Craving Scores. Craving was evaluated following each stimulus block in the scanner with the question “While focusing on the place/object, I craved a cigarette”. Participants responded with a bi-manual response pad on a scale of 1 (Do not agree) to 8 (Strongly agree). GLMM on mean in-scanner craving scores indicated significant main effects of CATEGORY (F2,140=24.84, p<.0001) and CUE (F1,140=499.45, p<.0001) and a CATEGORY x CUE interaction (F2,140=5.88, p=.0035). Post hoc paired t-tests revealed higher craving ratings in response to smoking versus nonsmoking cues for each cue category (all p’s<.001). In examining, cue-reactivity (craving in response to smoking minus nonsmoking cues), a main effect of CATEGORY (F2,54=7.53, p=0.0013) was observed for the change in craving in response to viewing smoking (relative to nonsmoking) cues. Post hoc paired t-tests indicate cue-reactivity in the personal environments condition was similar to the proximal cue condition (t=-1.23, p=0.224), but greater than that observed in the standard environment condition (t=2.74, p=0.008). 6 Figure S3. ROI Locations and Results. Table displays (1) ROI name, (2) ROIs viewed in two planes, (3) significant statistical findings and (4) graphs displaying findings separately for left and right hemisphere. P values are presented for all statistical tests. Tests were considered significant at α = .05 for hippocampal ROIS, all other ROIs were tested at α= .05/10 = .005. Legend as displayed in Figure S2. See Table S3 for effect sizes. Name ROI Left Statistics (α=.05) Left % Signal Change Right Statistics (α=.05) anterior hippocampus (aHPC) CAT: F=4.0, p=.0187 CUE: F=7.1, p=.008 CAT: F=7.8, p<.0005 CUE: F=4.1, p<.043 posterior hippocampus (pHPC) CAT: F=30.7, p<.0001 CUE: F=7.8, p=.0054 CAT: F=27.3, p<.0001 CUE: F=3.9, p=0.048 CATxCUE: F=3.7, p=.024 Name ROI Left Statistics (α=.005) Left % Signal Change Right Statistics (α=.005) amygdala (AMG) CUE: F=8.3, p=.0042 insula (INS) CAT: F=12.7, p<.0001 CUE: F=11.4, p=.0008 CATxCUE: F=6.9, p=.0011 CAT: F=21.3, p<.0001 CATxCUE: F=5.34, p=.005 CAT: F=23.2, p<.0001 CUE: F=8.4, p=.0039 CAT: F=15.4, p<.0001 precuneus (pc) Right % Signal Change CAT: F=8.7, p=.0002 ventral striatum (vSTR) medial prefrontal cortex (mPFC) Right % Signal Change CAT: F=122.1, p<.0001 CAT: F=129.4, p<.0001 7 Table S3: ROI Effect Sizes Effect sizes (Cohen’s d) are shown for smoking compared to nonsmoking stimuli for proximal cues as well as standard and personal environments for each of the ROIs in Figure S3. LEFT REGION Prox Stnd RIGHT Prsnl Prox Stnd Prsnl anterior hippocampus (aHPC) posterior hippocampus (pHPC) ventral striatum (vSTR) 0.23 0.13 0.29 0.28 0.24 0.26 0.40 0.46 0.40 0.31 0.25 0.02 0.19 0.15 0.28 0.13 0.55 0.59 amygdala (AMG) insula (INS) 0.26 0.19 0.16 0.01 0.59 0.90 0.28 0.11 0.14 0.26 0.40 0.59 medial prefrontal cortex (mPFC) 0.45 0.27 0.33 0.18 0.18 0.34 precuneus (pc) 0.20 0.38 0.14 0.10 0.29 0.12 8 Figure S 4. ICA Components z = -7 8 23 38 53 1 2 3 4 5 6 9 7 8 9 10 11 12 10 13 14 15 16 17 18 11 19 20 12 References 1. Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111(1-2):58-63. 2. Guo H, Song AW. Single-shot spiral image acquisition with embedded z-shimming for susceptibility signal recovery. J Magn Reson Imaging. 2003;18(3):389-95. 3. Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers' cue-reactivity. Exp Clin Psychopharmacol. 2008;16(3):207-14. 13