SCH4U1_05_11c_Salts as Acids or Bases
advertisement

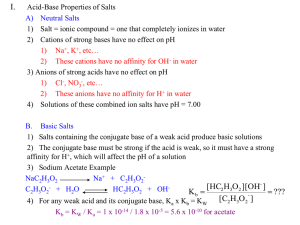
SCH4U1 Salts as Acids or Bases Salts Forming Neutral Solutions Many salts (ionic compounds containing an anion and cation) form neutral solutions. For example, sodium chloride always produces a solution of pH 7 at any concentration. This is because their ions react very little with the water they are dissolved in. As a rule, the cations of a strong base (i.e. Li+, Na+, K+, Rb+, Cs+) and the anion of a strong acid (Cl-, Br-, I-, NO3-) will not dramatically affect the pH of a solution. This is because the acid-base equilibria they are involved in overwhelmingly favour one side. For example: 100% Cl- (aq) conjugate base HCl (aq) acid + H2O Cl- + H2O no reaction H2O no reaction + H3O+ (aq) Likewise with the Na+ ion: Na+ + Other examples of neutral salts include KCl, LiBr and NaNO3. Salts as Weak Bases Many weak acids and bases occur as salts when in the solid state. For example, benzoic acid forms the conjugate base benzoate in aqueous solution: C6H5COOH (aq) + H2O C6H5COO- (aq) + H3O+ (aq) Ka = 6.5 x 10-5 Sodium benzoate (NaC6H5COO (s)) is the salt of this conjugate base. Since sodium salts dissociate fully and the sodium ion is unreactive, the important equilibrium is: C6H5COO- (aq) + H2O C6H5COOH (aq) + OH- (aq) Kb = Kw/Ka = 1.5 x 10-11 Salts as Weak Acids Ammonia (NH3) forms the conjugate acid ammonium (NH4+) in solution. This cation can form acidic salts such as ammonium chloride, NH4Cl (s). Since the chloride salt dissociates fully, the equilibrium is: NH4+ (aq) + H2O NH3 (aq) + H3O+ (aq) Ka = 5.8 x 10-10 Salts as Acids AND Bases Some salts are composed of an acidic cation AND a basic anion. For example, ammonium cyanide, NH4CN (s). This salt dissociates to yield NH4+ and CN- ions. These can each react with water: NH4+ (aq) CN- (aq) + + H3O+ (aq) H2O NH3 (aq) + H2O HCN (aq) + OH- (aq) Ka = 5.8 x 10-10 Kb = 1.6 x 10-5 What is the net effect? This will depend on the relative strengths of the weak acid and base involved. In this case, NH4CN will form a basic (alkaline) solution since the Kb > Ka value. Buffers A buffer is a solution containing a mixture of a weak acid and its conjugate base. Buffers are important because they maintain an approximately equal pH when a strong acid or base is added to them. Buffers are useful in chemistry to control the pH of a solution, such as in the EDTA titration. This lab used an ammonia/ammonium buffer to maintain a pH of 10. In biology, buffers are used to maintain the pH of human blood at 7.4. Changes in blood pH would have major effects on the chemical reactions required for healthy metabolism. Blood pH is maintained using a carbonic acid/bicarbonate buffer system. How a Buffer Works Consider the buffer system in blood: H2CO3 (aq) + H2O HCO3- (aq) + H3O+ (aq) Ka = 4.4 x 10-7 When a small amount of NaOH is added to this buffer, the hydronium drops and the equilibrium shifts right. However the shift in the equilibrium compensates for the decrease in hydronium. The overall effect is a change in the carbonate : bicarbonate ratio. The pH is only raised slightly due to this buffering action caused by the shift in the buffer equilibrium. Similarly, if a small amount of HCl was added, hydronium increases and the equilibrium shifts left to compensate. The pH will only decrease a small amount. Eventually, the buffering capacity of any buffer system will be exceeded and a base or acid will have a greater effect. Questions: Refer to your table of Ka and Kb values for any necessary constants. 1. Predict whether the following solutions are acidic, basic, or neutral. Provide explanations to support your predictions. a) ammonium phosphate, (NH4)3PO4(aq) (fertilizer) b) ammonium sulfate, (NH4)2SO4(aq) (fertilizer) c) Na2SO3(aq) (photographic developer) 2. a) Calculate the pH of a 0.50 mol/L potassium acetate (KCH3COO) solution. Ka = 1.8 x 10-5 for acetic acid b) Calculate the pH of a 0.30 mol/L ammonium nitrate (NH4NO3) solution. 3. a) Write an equilibrium reaction equation for each of the following buffer mixtures: i) NH3(aq) and NH4Cl(aq) ii) CH3COOH (aq) and NaCH3COO (aq) b) In what direction will each equilibrium shift if a small amount of HCl(aq) is added to the buffer? 4. A 1.0 L buffer is prepared from 1.5 mol of formic acid (HCOOH(aq)) and 1.5 mol of sodium formate (NaHCOO). Ka = 1.8 x 10-4 for formic acid. a) Write a Bronsted-Lowry equation for the buffer. b) Calculate the initial pH of this buffer solution. Since Ka > Kb assume the buffer solution will be less than pH 7. 5. Which of the following mixtures will form an effective buffer? Explain. a) HNO3(aq) and NaNO3(aq) b) NH3(aq) and NH4Cl(aq) c) HCl (aq) and NaOH(aq) d) citric acid and sodium citrate