File - Alex`s e
advertisement

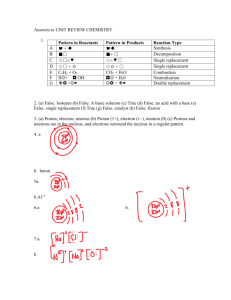
The Molecules Around Me Chemistry Project Alex Forkel (1): Food and nutritional supplements Name: Kirkland chewable vitamin C Purpose: Source of vitamin C Ingredients: Sorbitol, Sodium Ascorbate, Ascorbic Acid, Corn Starch, Magnesium Stearate, Silicon Dioxide, Sucralose, Yellow 6 Lake, Natural and Artificial Orange Flavors, Natural Orange Juice Powder, Lactose (Milk), Red 40 Lake. Compound(1): Name: Magnesium Stearate Molecular formula: Mg(C18H35O2)2 Organic or Inorganic: Organic because it has Carbon. Molecular structure: Summary: Magnesium stearate is a white substance, a powder that becomes solid at room temperature. Magnesium stearate melts at approximately 120 °C, but is not soluble in water, and is for the most part, considered safe for human consumption at levels below 2500 mg/kg per day. Compound(2): Name: Silicon Dioxide Molecular formula: SiO2 Organic or Inorganic: Inorganic because it does not contain any Carbon. Molecular structure: Summary: Silicon dioxide is a chemical compound that is an oxide of silicon. It has been known for its hardness since ancient times. Silicon is found most commonly in nature in the form of sand or quartz. Compound (3): Name: Sucralose Molecular formula: C12H19Cl3O8 Organic or Inorganic: Organic because it contains Carbons. Molecular structure: Summary: Sucralose is an artificial sweetener. Most of ingested sucralose is not broken down by the body, so it’s non-caloric. Sucralose is about 600 times as sweet as sucrose (table sugar), twice as sweet as saccharin, and three times as sweet as aspartame (the sweetener in gum). The most common brand names of sucralose-based sweeteners are Splenda, Sukrana, SucraPlus, Candys, Cukren and Nevella. Compound(4): Name: Sorbitol Molecular formula: C6H14O6 Organic or Inorganic: Organic because it contains Carbons. Molecular structure: Summary: Sorbitol is a sugar alcohol that the human body metabolizes very slowly. It can be obtained by reduction of glucose. Sorbitol can be found in apples, pears, peaches, and prunes. Compound(5): Name: Lactose Molecular formula: C12H22O11 Organic or Inorganic: Organic because it contains Carbons. Molecular structure: Summary: Lactose is a disaccharide sugar that comes from galactose and glucose that is most commonly found in cow milk. Lactose makes up around 2~8% of milk (by weight). (2): Healthcare and medicinal products Name: Ibuprofen Purpose: Pain reliever\Fever reducer Ingredients: Colloidal Silicon Dioxide, Corn Starch, Croscarmellose Sodium, Hypromellose, Iron Oxides, Microcrystalline Cellulose, Polyethylene Glycol, Polysorbate 80, Stearic Acid, Titanium Doxide Compound(1): Name: Corn Starch Molecular Formula: C27H48O20 Organic or Inorganic: Organic because it comes from corn, which contains Carbon. Anything that contains Carbon is organic. Molecular Structure: Summary: I read that they put Corn Starch in Ibuprofen to actually hold the tablet together. Some people add it to their homemade gravy to thicken it. It’s not exactly healthy, but like most things in this world, in small increments, it won’t harm you. Compound(2): Name: Croscarmellose Sodium Molecular Formula: C12H10Ca3O14.4H2O Organic or Inorganic: Organic because it contains carbon. Molecular Structure: Summary: Because it is soluble in water, but it must sit in water for a while before it breaks down, they put in in Ibuprofen to keep the tablets from breaking down too soon. If you took Ibuprofen on an empty stomach, (you have to take a lot for this to occur) you’d have a really upset stomach. So they do that to allow time. Compound(3): Name: Iron Oxide Molecular Formula: Fe2O3 Organic or Inorganic: Inorganic because it contains no Carbon. Molecular Structure: Summary: It is a combination of Iron and Oxygen (just in case you couldn’t tell) and it is what makes the Ibuprofen have it’s red color. There are a total of 16 known Iron Oxides. Most rust is a form of Iron Oxide. Compound(4): Name: Titanium Dioxide Molecular formula: TiO2 Organic or Inorganic: Inorganic because it DOES NOT contain Carbon. Molecular structure: Summary: Titanium dioxide is the naturally occurring oxide of titanium. When used as a pigment, it is called titanium white. It has a wide range of applications, from paint to sunscreen to food coloring. When used as a food colouring, it has E number E171. Compound(5): Name: Stearic Acid Molecular formula: C18H36O2 Organic or Inorganic: Organic because it contains Carbons and to be considered organic, a compound must contain Carbon. Molecular structure: Summary: Stearic acid is a saturated fatty acid with an 18carbon chain. It’s a waxy solid. The salts of stearic acid are called stearates, which is where the stearic in the name comes from. Stearic acid is one of the most commonly found (in nature) saturated fatty acids besides Palmitic Acid. (3) Personal hygiene and cleaning products Name: Crest 3D white toothpaste Purpose: To clean and significantly whiten teeth with results you can see in just 7 days. Ingredients: water, sorbitol, hydrated silica, disodium pyrophosphate, sodium lauryl sulfate, flavor, cellulose gum, sodium hydroxide, sodium saccharin, carbomer, xanthan gum, polyethylene, mica, titanium dioxide, blue 1 lake. Compound(1): Name: Water Molecular formula: H2O Organic or Inorganic: Inorganic because there’s no Carbon in it. Molecular structure: Summary: A water molecule is made of one oxygen and two hydrogen atoms that are bonded by covalent bonds. Water exists in a liquid crystal state near hydrophilic surfaces. Compound(2): Name: Hydrated Silica Molecular formula: SiO2 · nH2O Organic or Inorganic: Inorganic because it doesn’t contain any Carbon Molecular structure: Summary: Hydrated silica is a form of silicon dioxide, that contains a variable amount of water in the formula. It is also known as silicic acid, the term generally used for it when it’s dissolved in water. It’s found in nature as opal (which has been mined as a gemstone for centuries). It also tends to be sold for toothpaste. As soon as it’s dehydrated, the gel is used as a desiccant that we know as silica gel. It also tends to be used in many paints and varnishes. Compound(3): Name: Disodium Pyrophosphate Molecular formula: Na2HPO4 Organic or Inorganic: Inorganic because it does not contain Carbon. Molecular structure: Summary: Disodium hydrogen phosphate is sodium salt made of phosphoric acid. It is a white powder that’s soluble. Because of that, it is commercially sold as an anti-caking additive in powdered products. It is commercially available in both the hydrated and anhydrous forms. Compound(4): Name: Cellulose Gum Molecular formula: -CH2-COOH Organic or Inorganic: Organic because it contains Carbon. Molecular structure: Summary: “Carboxymethyl cellulose (CMC) or cellulose gum, is a cellulose derivative with carboxymethyl groups bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose.” (Wikipedia.org) Compound(5): Name: Xanthan Gum Molecular formula: C35H49O29 Organic or Inorganic: Organic because it contains Carbons. Molecular structure: Summary: “Xanthan gum is typically used as a food additive and rheology modifier, commonly used as a food thickening agent (in salad dressings, for example) and a stabilizer (in cosmetic products, for example, to prevent ingredients from separating). It is produced by the fermentation of glucose, sucrose, or lactose. After a fermentation period, the polysaccharide is precipitated from a growth medium with isopropyl alcohol, dried, and ground into a fine powder. Later, it is added to a liquid medium to form the gum.” (Wikipedia.org) Summary: Overall, I learned that there are things that you can learn stuff about in my own house and it’s super quick and easy. It’s just Google. That’s way cool. Also, I’d never tasted a chewable vitamin C before I pulled it out of my cupboard to use. So that was cool. I always just took the liquid. They’re really good. I think what I learned that most relates to chemistry is that the FDA DOES NOT approve of a lot of things for us to consume that are put in ibuprofen and vitamins (I learned this from other’s projects). I think it would’ve been really cool if we’d had some way to look at other’s projects because I learned probably just as much reading theirs as I did probably doing mine.