Mitchell Minerals Makeup
advertisement

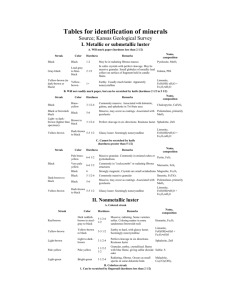
Mary Beth Mitchell GEOL 113 Minerals lab Spring 2014 Minerals 1. Describe the following mineral properties: Color: many minerals have a distinctive color that can be used for identification. Although color is often a way you can identify a mineral it alone is not reliable as a single way to identify them. Streak: Streak is the color of the mineral in powdered form. Streak shows the true color of the mineral. Because streak is a more accurate illustration of the mineral’s color, streak is a more reliable property of minerals than color for identification. Cleavage: Minerals tend to break along lines or smooth surfaces when hit sharply. Different minerals break in different ways showing different types of cleavage. Fracture describes the quality of the cleavage surface. Most minerals display either uneven or grainy fracture, conchoidal (curved, shell-like lines) fracture, or hackly (rough, jagged) fracture. Luster: Luster is the property of minerals that indicates how much the surface of a mineral reflects light. The luster of a mineral is affected by the brilliance of the light used to observe the mineral surface. The most common types of lusters are metallic and nonmetallic. Harness: Hardness is one of the easier properties of minerals to use for identifying a mineral. Hardness is a measure of the mineral’s resistance to scratching.. Softer minerals can be scratched by harder minerals because the forces that hold the crystals together are weaker and can be broken by the harder mineral Reaction with hydrochloric acid: hydrochloric acid is one way to quickly identify arbonate minerals and rocks. Any rock with calcite reacts quickly with large bubbles. Reactions are very slowly, and pure must be powdered to get even a weak reaction, very fine, slow bubbles. Mary Beth Mitchell GEOL 113 Minerals lab 2. Spring 2014 How would you distinguish between the following mineral pairs: Quartz vs. Calcite Calcite vs. Feldspar Quartz vs. feldspar Augite vs. Hornblende Biotite vs. muscovite Hematite vs. limonite Quartz - contains silicon dioxide - hardness of 7 - does not dissolve in acids - weak cleavage in 3 directions Calcite - rhombohedra cleavage - white - good cleavage - hardness of 3 Quartz - consist of silicon and oxygen - colorless or appears clear, gray purple, brown - poor cleavage - hardness of 7 Augite -90 degree cleavage -dark green -good cleavage Biotite -black sheets -good/excellent cleavage -2.5 / 3 hardness Hematite - red streak can very in color - poor cleavage Calcite -contains calcium carbonate -hardness of 3 -dissolves in acids -perfect cleavage in 3 directions Feldspar - elongated cleavage -more commonly found -contains aluminum and silica ion -light colored -excellent cleavage hardness of 6 Feldspar more commonly found -contains aluminum and silica ion -light colored -excellent cleavage hardness of 6 Hornblende 60- 120 degree cleavages -dark gray/black - good cleavage muscovite -clear silvery or coppery color sheets -fine cleavage -2 / 2.5 hardness limonite -yellow-brown streak -submetallic -poor cleavage Mary Beth Mitchell GEOL 113 Minerals lab Spring 2014 - 6 /6.5 hardness