View/Open
advertisement

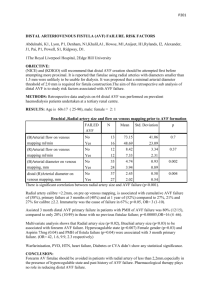
Mufty 1 Title page Proactive surveillance approach to guarantee a functional arteriovenous fistula at first dialysis is worth. Hozan Mufty, MD (1), Kathleen Claes, MD, PhD (2) , Sam Heye, MD,PhD (3), Inge Fourneau, MD, PhD (1) (1) Department of Vascular Surgery, University Hospitals Leuven, Leuven, Belgium (2) Department of Nephrology, University Hospitals Leuven, Leuven, Belgium (3) Department of Interventional Radiology, University Hospitals Leuven, Leuven, Belgium Corresponding author: Fourneau Inge, MD, PhD Dept. of Vascular Surgery, University Hospitals Leuven Herestraat 49, 3000 Leuven, Belgium Telephone:00 32 16 34 68 50 Fax: 00 32 16 34 68 52 Inge.Fourneau@uzleuven.be Mufty 2 Abstract Purpose To evaluate the impact of a proactive surveillance program on functional access rate at the time of first dialysis. Methods In January 2010 a proactive surveillance program to intercept failures to mature was set up at the University Hospitals Leuven. Patients receiving an AVF for pre-dialysis end stage renal disease between January 2010 and May 2013 were retrospectively analyzed. The primary end-point was a functional AVF at first dialysis. Also AVFassociated complications and reinterventions were analyzed. Furthermore, primary, assisted primary and secondary patency rates were estimated using Kaplan-Meier survival curves. Results One hundred sixty four patients were included in the study. Patients were followed until first dialysis. Median follow-up time was 287 days (interquartile range, 108 to 551). During follow-up 40 patients (24.4%) needed one or more additional interventions, resulting in 60 reinterventions. Ten patients needed dialysis within the minimal accepted maturation period of the AVF (four weeks). Of the 154 patients that could await the maturation period of the AVF, 145 (94.2%) appeared ready for use at the time of dialysis or at the end of the study period. In 34 of them (22%), this was thanks to one or more interventions during follow-up. Mufty 3 Conclusion A dedicated surveillance program of patients with AVFs in the pre-dialysis stadium detects failure to mature. Close coaching and proactive intervention can aid the patient in his own “fistula first ‘project. Key words: Arteriovenous fistulas – Maturation- Dialysis- Functional outcome – End-stage renal disease Mufty 4 Introduction Worldwide, the number of patients developing end-stage renal disease (ESRD) has increased linearly over the past decades. Diabetes mellitus and arterial hypertension are the leading causes of ESRD. According to the 2013 United States Renal Data system annual data report, the majority of patients with ESRD are treated with hemodialysis. [1] In this patient group, a well-functioning vascular access is of utmost importance. Autologous arteriovenous fistula (AVF) is the primary choice for vascular access. [2, 3] At one year, AVF has a comparable cumulative survival rate as to arteriovenous grafts (AVG), but requires less interventions and has a lower infection rate. [4] Given these findings, every institution attempts to achieve a high AVF placement rate in patients with ESRD. However, at time of dialysis initiation many AVFs appear not usable due to a lack of maturation, inability to needle for anatomical reasons or thrombosis. [4] As this happened in spite of regular medical follow-up at the nephrologic outpatient clinic, we started a dedicated proactive follow-up program for patients after AVF creation in the pre-dialysis stage. The aim of the program was to optimize the functional access rate at first dialysis. In this study we investigate how often a failing AVF could be picked up and remediated, resulting in a functional AVF at the time of dialysis initiation. Mufty 5 Patients and methods 1. Patient population Between January 2010 and May 2013, 215 patients were seen at a dedicated clinic for AVF in the University Hospitals Leuven, Leuven, Belgium. A total of 167 patients presented prior to AVF creation in a predialysis stage and are the scope of this study. Of these, 166 patients presented with ESRD. One patient with hereditary hemochromatosis was given an AVF for bloodletting. Three patients were not followed at our dedicated clinic for AVF as they were referred to an external nephrology department immediately after surgery and were therefore excluded from the study. This resulted in 164 patients meeting the inclusion criteria of the study. The study adhered to the principles of the declaration of Helsinki and was approved by the Ethics Committee of the University Hospital of Leuven. Data were retrieved retrospectively from the electronic patient files. 2. Dedicated surveillance program Surgery was performed by one single surgery unit. Prior to AVF creation, the site of AVF was determined on a clinical basis by the vascular surgeon at the outpatient clinic. Criteria for choosing the site were based on caliber and quality of the veins, previous surgery, patient comorbidities and patient preference. In case of major risk factors (e.g. female, eldery), an upper arm AVF was preferred. When access opportunities were equal in both arms, the non-dominant arm was preferred over the dominant arm. Preoperative assessment by physical examination was performed in all patients Mufty 6 to evaluate the patency and diameter of the superficial venous system and to detect indirect signs of central venous occlusive disease and evaluate the radial pulse. All patients had palpable radial pulsations preoperatively. Adjuvant duplex ultrasound or phlebography were performed when the physical examination was inconclusive. In 17 patients additional duplex (n=3) or phlebography (n=14) were performed because of an unclear clinical venous anatomy. A three month maturation period was intended before needling. Immediately postoperatively, the patient was instructed to palpate the thrill over the AVF on a daily basis and to come to the emergency department the same day in case of disappearance of the thrill. Every patient received an information leaflet. Also the general practitioner was informed. Patients were seen at a dedicated outpatient clinic at 2 weeks, 6 weeks, 3 months, 6 months and 12 months postoperatively, both by the vascular surgeon and a dialysis nurse. AVF patency, maturation and functionality were checked by physical examination of the AVF and ultrasound. Physical examination of the AVF was performed in two steps. The anastomosis was checked with control of bruit by auscultation and thrill by palpation. The body of the fistula was checked by the presence of pulse augmentation, by searching for accessory veins and by the elevation test as described by Malovrh. [5] The efferent vein was checked for wall thickness, straightness and depth by ultrasound. Continued AVF monitoring also occurred in patients with satisfactory physical examinations. In case a stenosis was suspected, additional duplex and/or fistulography was performed. If indicated, a salvage Mufty 7 intervention was performed or, when a salvage procedure was not possible, a new AVF was created. All interventional endovascular procedures were performed by one interventional radiology service. All surgical procedures were performed by the same surgical team that created the original AVF. The patients costs weren’t taken into account in our study because every intervention and outpatient clinic is reimbursed by the Belgian health insurance. The primary end point of our study was the presence of a functional AVF at time of first dialysis. Also AVF-placement-associated complications and reinterventions were analyzed. Furthermore, primary, assisted primary and secondary patency rates were estimated using Kaplan-Meier survival curves. 3. Definitions Primary patency (intervention-free survival) is defined as the interval between the time of AVF placement and any intervention designed to maintain or reestablish patency, access thrombosis, or the time of patency measurement. Assisted primary patency (thrombosis free survival) is defined as the interval between the time of AVF placement and access thrombosis or the time of patency measurement, including intervening manipulations designed to maintain the functionality of a patent access. Secondary patency is defined as the interval between the time of AVF placement until access abandonment, thrombosis, or the time of patency measurement including intervening manipulations designed to reestablish functionality in thrombosed access. [6] 4. Statistical Analyses Mufty 8 Results are shown as median with interquartile range or means ± standard deviation unless otherwise specified. Kaplan-Meier survival analysis (WinSTAT® for Microsoft® Excel) was used to calculate patency rates. Results One hundred sixty four patients were included in the study. In 163 the AVF was created because of ESRD. One female patient was given an AVF for the treatment of hereditary hemochromatosis. Table I demonstrates the clinical characteristics of the patients. The AVF was created in the non-dominant hand in 107 patients (65.2%). One hundred and four AVFs (63%) were created at the wrist, 60 patients (37%) received their AVF at the elbow. Table II provides an overview of the various AVF constructions. One hundred and fifty-one AVFs (92%) could easily be gauged with a coronary dilator of 2.5 mm. Although the vein diameter in the remaining 13 patients was smaller, the AVF was created for a lack of alternative options. This also occurred in younger patients to maximize the use of the available veins. All operations were uncomplicated. Postoperatively, 107 patients (65%) were on antiplatelet medication or anti-coagulation therapy (acetylsalicylic acid, clopidogrel or warfarin). The median time until first control was 17 days (interquartile range, 12 to 24). Median follow-up time was 287 days (interquartile range, 108 to 551). Twenty-six Mufty 9 patients (15.8%) died due to procedure-unrelated causes after a mean of 462 days ± 278 (range 80-981 days; median 384 days) after AVF placement. Criteria for further exploration at the time of check-up were absent or discrete thrill and /or bruit, high frequency overtone, positive elevation test, a persistent small diameter of the efferent vein and a deep or tortuous efferent vein. During follow-up 40 patients (24.4%) needed one or more additional interventions. In total 60 reinterventions were performed. Twenty-seven patients (16.4%) underwent one additional procedure, 10 patients (6.0%) underwent two additional procedures and two patients (1.2%) four. One patient (0.6%) underwent five procedures, including a new AVF at the third intervention. In 31 patients (31/40; 77.5%), 40 reintervention were needed because of failure to mature. This results in an average of 1.29 interventions per non-maturing AVF. Nine patients (9/40; 22.5%) needed reinterventions (n=20) because of dysfunction after initial successful maturation. Numbers and types of intervention are given in Table III. Patients underwent their first reintervention after a mean of 109 days ± 116 after surgery (range 2 – 510 days; median 68 days). Ten patients needed dialysis within the minimal accepted maturation period of the AVF (four weeks). These patients all received a central venous tunneled cuffed catheter and were consequently excluded for further follow-up in the study. Of the 154 patients that could await the maturation period of the AVF, 145 of the AVF appeared ready for use at the time of dialysis or at the end of follow-up, resulting in a Mufty 10 functional outcome of 94.2%. In 34 of them (22%), this was thanks to one or more interventions during follow-up. In twenty patients (20/34) a new AVF was created. Seven patients (7/20) who received a new AVF, needed additional intervention for adequate maturation. In one patient out of this latter group, an AVG was created eventually. In 9 patients (5.8%) no functional AVF was available at initiation of dialysis despite intensive follow-up. Data for AVFs at level of the wrist and at the elbow are given in table IV. Ninety-five percent (19/20) of the newly created AVFs were created because of failure of the AVF at level of the wrist. At the end of the study period dialysis was already initiated in 85 patients; in 66 of them dialysis could be initiated through a functional AVF after a mean of 305 days (range 40-979 days; median of 243 days) after creation of the AVF. Seventy-nine patients with a mature AVF had not yet started dialysis. Kaplan-Meier estimates for primary patency, assisted primary patency and secondary patency rate at one year were 67.7% ± 4.2, 82.9% ± 3.4 and 84.6% ± 3.3, respectively (Figure 1). Discussion The aim of our dedicated surveillance program was to optimize the functional access rate at first dialysis. We succeeded to deliver a functional outcome rate of 94.2%. We didn’t change our technique to achieve this results. We did it by proactive intervention Mufty 11 in a rapid fashion to rescue the AVF and by coaching the patient and his surroundings in the value of this “fistula first project”. The National Kidney Foundation-Kidney Disease Outcome Quality Initiative (NKFKDOQI) guidelines advise AVF as first access option in long-term dialysis access, followed by AVG. The use of catheters is discouraged. The NKF-KDOQI guidelines encourage appropriate planning for the initiation of dialysis therapy with a permanent access in the pre-dialysis stage. [2, 3] In an effort to improve outcomes, the rates of autologous AVF have increased in the US in the last few years. In Europe, its use is even significantly more common. Surgical training, surgical techniques and postoperative management of AVFs are considered to be important determinants of AVF outcome. A lower event rate, better AVF salvage techniques and higher AVF survival rates are observed in Europe. In Europe, the 1-year AVF survival rate is 83% versus 63% in the United-States. [7] Timely creation of an AVF is important but many AVFs fail to mature adequately or develop dysfunction awaiting the first dialysis and patients still end-up with a dysfunctional AVF at time of dialysis. Consequently, there is a need for interception of these AVFs in due time and for remediating them. Given these findings and experiencing this in our own patients in spite of regular medical follow-up at the nephrologic outpatient clinic, we decided to initiate a proactive follow-up program on a specialized AVF outpatient clinic for patients with an AVF in the pre-dialysis stage to optimize the presence of a functional AVF at time of dialysis. Mufty 12 The fully matured AVF has ideally a superficial, large-caliber efferent vein that allows a high flow and repeated large-bore punctures. Therefore, several steps have to be completed. The basis is a good surgical construction, leading to vascular remodelling. AVF dilatation and vessel wall hypertrophy are end points. Unfortunately, a substantial fraction of these AVFs fail in their maturation process. [8] This is due to an interaction between adverse vascular remodeling and hemodynamic forces such as wall shear stress and transmural or circumferential pressure. [9] A maturation failure rate ranging from 37 to 61% has been documented in recent studies. [10, 11, 12] The DOPPS study advises a 95 days maturation time [7], though generally a shorter time is advised ranging from 3- 6 weeks. [2, 3] AVF thrombosis is easily picked up at the regular outpatient follow-up, but failure to mature is much harder to detect. We achieved a failure rate of only 5.8 % with an average of 1.29 interventions / nonmaturing AVF. This is lower compared to published data. Allon et al. compared primary AVF failure rates of older studies (20-25 years ago) with more recent studies and found a higher failure rate in the latter one (about 10% vs. 20-50%). They attributed this to a more liberal selection in creation of AVFs nowadays. The population of patients with ESRD is becoming older, is more often diabetic with a higher co-morbidity rate. [4] A rate of 1.7 interventions/AVF has been observed in literature. [13] The first diagnostic step in the detection of AVF abnormalities is physical examination. This should include inspection (erythema, swelling, gangrene, change of size over time), palpation (intravascular pressure along veins, segmental differences in quality of thrill, skin temperature, pulsation, pain caused by finger pressure) and auscultation Mufty 13 (typical low-frequency bruit with systolic and diastolic components). Ferring et al recently published data concerning early postoperative AVF screening at four weeks. Presence of a thrill and vein diameter ≥ 5mm are sensitive predictors ( 96% and 83% respectively) for future dialysis use. [13] When physical examination isn’t enough to deliver a definite diagnosis, further diagnostic steps should be undertaken by duplex ultrasound as a preferable first and angiography as a next step. [3] In our center 41.4% of reinterventions required prior duplex ultrasound investigation. In a retrospective study, Singh et al. controlled the value of ultrasonography in triaging immature AVFs and found an increase of 47% in maturation to AVFs that where usable for dialysis. [15] We did not find any similar studies focusing on a surveillance approach of maturing AVFs exclusively. In 2007, Barone et al. published their results of a dedicated hemodialysis arteriovenous clinic to provide preoperative evaluation and postoperative follow-up. Patients in this retrospective study had a follow-up period of six months, consequently including both immature AVFs and AVFs in use. They had a final AVF use rate of approximately 85%. Of all AVFs in their study, 38% matured without any intervention. The remaining 62% underwent an average of 2.2 +/- 0.3 early interventions/dysfunctional AVF. [16] Another study of Berman et al. published in 2001 focused on an aggressive work-up to evaluate the impact of secondary procedures to facilitate maturation of AVF and optimize their use as hemodialysis access. They achieved a functional access in 90% of the patients and an actuarial primary and assisted primary patency rate at one year of Mufty 14 78% and 93%, respectively. This study also included both immature AVFs and AVFs already in use. [17] Many monitoring and more time-consuming surveillance techniques have been reported on in literature. However, the sample size of these studies are too small to demonstrate any beneficial effect. Currently employed monitoring techniques include physical examination, review of routine laboratory studies, difficulties in cannulation or achieving hemostasis after cannulation, recirculation, measurement of dynamic venous pressure. Surveillance techniques on the other hand include access flow measurement, duplex ultrasound, direct or derived static pressure. Only limited techniques are available for detection of non-maturing AVF in a pre-dialysis stage. [18] This implies a need for more reliable control parameters in this subgroup. The Hemodialysis Fistula Maturation (HFM) study is a large multicenter prospective epidemiologic study in progress in the United States to evaluate mechanical hypotheses, to identify clinical practices associated with AVF maturation, to establish targets for new therapeutic interventions and to assess the predictive value of early indicators of AVF outcome. [19] Repeated interventions to promote AVF maturation are associated with an increase in interventions to maintain access patency once dialysis has started. These repeated interventions are also associated with a decreased cumulative AVF survival rate in the long term.[20] The question remains whether these reinterventions are worth or whether these salvaged AVFs should be left untouched and be considered as complete treatment failures? We can compare our data with data of a recently published study Mufty 15 by Verest et al in Acta chirurgica Belgica. Between January 2005 and December 2009, 344 patients received a AVF in the University Hospitals Leuven by the same surgeon as in our study. When we only consider the group of patients still in a predialysis stage (n=78) after six months of follow-up, 27% (21/78) underwent additional intervention. Twenty-two percent of the patients (17/78) received a new fistula whom 15 (19%) without preceding intervention on the failed AVF. [21] In our present study, only 12.9% (20/154) of the patients received a new AVF as many of the dysfunctional AVFs could be solved. Twenty-four percent of the patients needed one or more additional interventions. In the study of Verest et al, 1-year primary and secondary patency rate were 64% and 77% respectively, whereas in the present study a 1-year primary patency of 67.7% and secondary patency of 84.6%.is achieved. We managed to achieve a mature AVF ready for use in 94% of patients. Our dedicated surveillance program was led by a surgeon skilled in proactive interventions and our patient was taught to recognize a well functioning AVF. By taking enough time for every patient, this study turned out into a successful surveillance program with good results in which we avoid the additional morbidity (infection and thrombosis) of tunneled cuffed venous catheters. [3] Conclusion With this strict surveillance program, a functional success rate of 94.2% was achieved, in 22% of AVF, this was thanks to one or more interventions during follow-up. We suggest that the implementation of a dedicated surveillance program of patients with Mufty 16 AVF in a pre-dialysis stage is essential for every dialysis unit as failures to mature are often not picked up at a regular medical follow-up. Disclosures There are no disclosures List of figures: Figure 1: Kaplan-Meier survival analysis of the first event-free survival (primary patency), assisted primary patency and AVF survival (secondary patency) Mufty 17 Table I: Clinical characteristics of patients Clinical characteristics N AVF localization Wrist (N=105) Age (Years): Mean ± SD (range; median) Elbow (N=59) 65± 14.4 63±14.8 70±12.3 (21-89; 68 ) (21-86; 66) (27-89; 73) 97 (59%) 70 (67%) 27 (46%) Female: N (%) 67 (41%) 35 (33%) 32 (54%) BMI: mean ± SD (range; median) 26.8 ± 5.7 26.9±5.9 26.7±5.3 (14.5 – 54.9; 25.6) (14.5-54.9; 25.6) (17.5-46.8;25.8) 28 (17%) 20 (19%) 8 (13%) Stopped > 10years: N (%) 25 (15%) 17 (16%) 8 (14%) Stopped < 10years: N (%) 14 (9%) 8 (8%) 6 (10%) Never smoked: N (%) 97 (59%) 60 (57%) 37 (63%) 13 (8%) 8 (8%) 5 (9%) 50 (30%) 35 (33%) 15 (25%) Peripheral arterial disease: N (%) 49 (30%) 29 (28%) 20 (34%) Previous AVF: N, (%) 4 (2%) 2 (2%) 2 (3%) Cause of ESRD: Diabetes: N (%) 41 (25%) 29 (28%) 12 (20%) Glomerulonephritis/vasculitis: N (%) 31 (19%) 22 (21%) 9 (15%) Hereditary/congenital: N (%) 20 (12%) 13 (12%) 7 (12%) Tubulo-interstitial: N (%) 10 (6%) 5 (5%) 5 (9%) Hypertensive/vascular: N (%) 30 (18%) 21 (20%) 9 (15%) Miscellaneous: N (%) 4 (3%) 1 (1%) 3 (5%) Unknown: N (%) 27 (17%) 13 (12%) 14 (24%) Gender: Male: N (%) Smokers: Active: N (%) Diabetes Mellitus type 1: N (%) type 2: N (%) Mufty 18 Table II : Summary of AVF constructions Level Construction N (%) wrist Radio-cephalic AVF (RCAVF) 104 (63) elbow Radio-cephalic AVF (RCAVF 19 (12) Radio-median cubital AVF 16 (10) Brachio-basilic AVF (BBAVF) 9 (5) Brachio-cephalic AVF (BCAVF) 7 (4) Brachio-median cubital AVF 5 (3) Radio-basilic AVF (RBAVF) 3 (2) Ulno-median cubital AVF 1 (1) Mufty 19 Table III: Numbers and types of reintervention Type of intervention Endovascular % N 30.0 18 Angioplasty 17/18 Thrombectomy & 1/18 angioplasty OPEN 70.0 42 Thrombectomy 7/42 Superficialization & 7/42 lateralization Branch ligation 4/42 Re-anastomosis 3/42 New AVF 20/42 PTFE AV-loop 1/42 Mufty 20 Table IV: Data for AVFs at level of the wrist and at the elbow AVF lokalisation Wrist: N (%) Elbow: N (%) Start dialysis with catheter 7/98 (7.1%) 2/56 (3.5%) AVF ready for dialysis at end of follow-up 91/98 (92.8%) 54/56 (96.4%) Creation of new AVF after first event 15/98 (15.3%) 0/56 (0%) Creation of new AVF (total) 19/98 (19.3%) 1/56 (1.7%) Patients with need for HD within normal accepted maturation period were omitted in these data Mufty 21 References 1. 2013 United States Renal Data System (USRDS) annual data report. 2. Tordoir J, Canaud B, Haage P et al. EBPG on Vascular Access. Nephrol Dial Transplant. 2007; 22 Suppl 2:ii88-117. 3. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006; 48 Suppl 1: S176-247. 4. Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 2002; 62: 1109-24. 5. Malovrh M. Postoperative assessment of vascular access. J vasc access 2014; 15 (Suppl 7): S10-S14. 6. Sidawy AN, Gray R, Besarab A et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 2002; 35: 603-10. 7. Pisoni RL, Young EW, Dykstra DM et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 2002; 61: 305-16. 8. Saad TF. Management of the immature autogenous arteriovenous fistula. Vascular 2010; 18: 316-24. 9. Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P. Biology of arteriovenous fistula failure. J Nephrol 2007; 20: 150-63. 10. Dember LM, Beck GJ, Allon M et al. Dialysis Access Consortium Study Group. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008; 299: 2164-71. 11. Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ; CIMINO study group. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 2008; 3: 714-9. Mufty 22 12. Schinstock CA, Albright RC, Williams AW et al. Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol 2011; 6: 1996-2002. 13. Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol 2006; 17: 807-13. 14.Ferring F, Henderson J, Wilmink T. Accuracy of early postoperative clinical ultrasound examination of arteriovenous fistulae to predict dialysis use. J Vasc Access 2014; 15:291-297. 15. Singh P, Robbin ML, Lockhart ME, Allon M. Clinically immature arteriovenous hemodialysis fistulas: effect of US on salvage. Radiology 2008; 246: 299-305. 16. Barone GW, Wright CF, Krause MW et al. Hemodialysis access success: beyond the operating room. Am J Surg 2007; 194: 668-71. 17. Berman SS, Gentile AT. Impact of secondary procedures in autogenous arteriovenous fistula maturation and maintenance. J Vasc Surg 2001; 34: 866-71. 18. Kumbar L, Karim J, Besarab A. Surveillance and monitoring of dialysis access. Int J Nephrol 2011;Nov 22 (Epub ahead of print). 19. Dember LM, Imrey PB, Beck GJ et al. Hemodialysis Fistula Maturation Study Group. Objectives and Design of the Hemodialysis Fistula Maturation Study. Am J Kidney Dis 2014; 63: 104-112. 20. Lee T, Ullah A, Allon M et al. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 2011; 6: 575-81. 21. Verest S, Logghe P, Claes K , Kuypers D, Fourneau I. Can a policy of careful clinical examination and preference towards upper arm fistulae in high risk patients improve maturation rates of native arterio-venous fistulae? Acta Chir Belg 2014; 114: (ahead of print).