Unit 5/6 - Carbon Chemistry Uniqueness of Carbon 1) 4 valence
advertisement

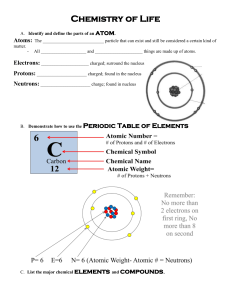
Unit 5/6 - Carbon Chemistry Uniqueness of Carbon 1) 4 valence electrons allowing each carbon atom to bond to 1 – 4 atoms. (S 1) 2) Can form ionic bonds with metals and covalent bonds with nonmetals and H. (S 2) 3) Can bond to other carbon atoms to form chains and rings. (S 3) 4) Carbon chains can be straight or branched. 5) Can form coordinate crystals made up exclusively of carbon atoms. (S 4) 6) Bonds between carbon atoms can involve: (S 5) 1 pair of electrons – Single bond 2 pairs of electrons – Double bond 3 pairs of electrons – Triple bond These properties allow carbon to be the central element in a wide variety of compounds. Well over 6 million different carbon based compounds have been found or made. A special area of chemical study called organic chemistry is dedicated to the study of these carbon based compounds. Organic Compound – any type of chemical compounds whose molecules contain carbon, with the exceptions of carbides, carbonates and oxides. Inorganic compounds – compounds that are not based on carbon. All organic compounds are held together by covalent bonds with most organic compounds involving bonds between Carbon and Hydrogen. Hydrocarbon – a compound made up of carbon and hydrogen. Ex – Gasoline (mostly octane C8H18) Hydrocarbons are classified as: (S 6) 1) Saturated – all carbon to carbon bonds are single bonds 2) Unsaturated – one or more carbon to carbon bond is a double or triple bond 3) Substituted – one or more hydrogen is replaced by a different element, or group of elements (S 7) Groups that are typically found in substituted hydrocarbons include: -OH which makes the compound an alcohol. (S 8) -COOH which makes the compound an acid. (S 9) -NH2 which is called an amino group. (S 10) In biological systems have amino groups and acid groups combined in a structure called an amino acid. Amino acids are the structural basis of proteins. (S 11) Properties of simple Hydrocarbons (no substitutions) include: 1) Nonpolar bonds 2) Low melting point and boiling point (Low attractions between molecules) 3) Flammable making them useful as fuels 4) Do not usually dissolve in water 5) Tend to be Alcohol Soluble Substitutions will alter the properties depending on the element or group that is inserted. The naming of a hydrocarbon is based on: 1) Number of carbon atoms in the longest chain 2) Type of bond The number of carbons is indicated by a prefix: 1 = meth2 = eth3 = but4 = prop5 = pent6 = hex7 = hept8 = oct9 = non 10 = decSuffixes are used to indicate the type of bond present, hydrocarbons with a: Single bond end in –ane Double bond ends in –ene Triple bond ends in –yne Example: (S 12) -C-C-C- Butane -C-C-C=C- Propene -C-C-C-C-C≡C- Hexyne