Unit 4: Chemical Reactions Binder Bulletin
advertisement

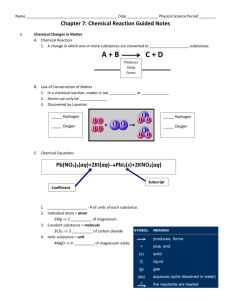
Binder Bulletin and Study Guide UNIT 4: CHEMICAL REACTIONS The Big Picture… When a chemical reaction occurs you will find that the amount of matter you end with will equal the amount of matter you started with. The process of a chemical reaction or change is illustrated though equations that consist of chemical formulas and other symbols. It is important that the equation or reaction be “balanced” because you cannot create or destroy matter during this process. Bonding two or more elements together creates a compound that is new in both its structure and properties. Chemical reactions occur when molecules rearrange by breaking old bonds, forming new ones or both. Bonds can also be formed between atoms. We call this rearrangement of bonds a chemical reaction. These chemical reactions change the properties of an object and give off signs that a chemical change has indeed been made. What happens to wood when it burns? If matter is not created or destroyed, what happens to it? How can you prove that cooking is a chemical reaction? Prerequisite Knowledge…You should be able to: 1) Use symbols to represent the first 20 elements 2) Use the Periodic Table as a tool to obtain information 3) Interpret a chemical formula 4) Use basic computational skills (add, subtract, multiply, divide) 5) Follow safety procedures in a lab environment 6) Explain why the properties of compounds are different from the elements they are composed of Suggested Resources… Homework and Classwork Assignments Laboratory Activities and Quizzes Textbook pages: Chapter 14: p. 388-391 (Forming New Substances) Chapter 14: p. 392-397 (Chemical Formulas and Equations) Websites: http://education.jlab.org/elementbalancing/index.html http://www.elmhurst.edu/~chm/vchembook/105Achemprop.html http://www.elmhurst.edu/~chm/vchembook/104Aphysprop.html Key Terms: Chemical change Mass Chemical equation Matter Chemical formula Physical Change Chemical property Physical Properties Chemical reaction Precipitate Coefficient Products Compound Reactants Element Subscript Endothermic reaction Volume Exothermic reaction Yield sign Law of Conservation of Mass Directions: Use this information as a general reference tool to guide you through this unit. Don’t hesitate to ask your teacher for help! By the conclusion of this unit, you should be able to explain each main idea below in your own words with supporting details: ______1. The Law of Conservation of Matter states that matter is not created or destroyed in the universe but changed to different forms. ______2. Reactants are the starting molecules or elements that participate in a chemical reaction and are found on the left side of the chemical reaction. ______3. The arrow pointing left in a chemical reaction (yield sign) indicates that a chemical reaction is taking place. ______4. Products are molecules that are formed in a chemical reaction and are found on the right side of the chemical equation. ______5. The number of each atom on the reactants side of a chemical equation has to be equal to the number of that atom on the product side due to the Law of Conservation of Matter. ______6. In a chemical reaction, a coefficient is a number that is placed in front of a chemical symbol or formula and represents the number of molecules to be used in a balanced equation. ______7. In a specific chemical formula, subscripts cannot change because that would change the compound being used. ______8. The only way to increase the amount of a specific atom or molecule is to change the coefficient in the chemical formula. ______9. Chemical reactions cause a chemical change which will result in new substances forming. ______10. A chemical change occurs when one or more substances are changed into new substances that have properties different from the old one. ______11. During a chemical reaction, bonds are broken, rearranged, and formed to create new bonds. ______12. An exothermic reaction is a reaction that gives off heat. (Exo=Exit) ______13. An endothermic reaction is a reaction that absorbs heat. (Endo=inside). By the conclusion of this unit, USING THE MAIN IDEAS ABOVE AND INFORMATION FROM CLASS, you should be able to complete the following tasks: ______1. Analyze the mass of substances before and after a chemical reaction to draw conclusions about the Law of Conservation of Mass. ______2. Correctly use the vocabulary involved with balancing equations. ______3. Evaluate a chemical equation to identify if an equation is balanced or unbalanced. ______4. Justify why equations need to be balanced ______5. Describe the five signs of a chemical change: a. Precipitate (solid formation) b. Gas formation c. Energy change d. Color change e. Odor change ______6. Demonstrate what is happening to a chemical bond during a chemical reaction