Investigation of the metal – non metal transition in metal ammonia
advertisement

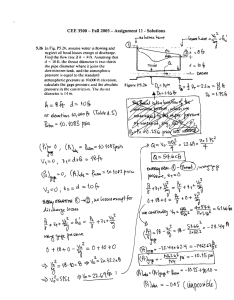
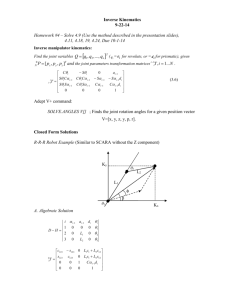
Investigation of the metal – non metal transition in metal ammonia solutions: an x-ray study + P. Giura, #R.Angelini , OC. A. Burns, *M. Dimichiel, *C. Henriquet, *G. Monaco, *F.Sette +IMPMC, Université Pierre et Marie Curie, Paris 6, UMR7590, 140 Rue de Lourmel, Paris F-75015, France, #CNR-INFM CRS-SOFT, Universitá di Roma “La Sapienza,” Piazzale Aldo Moro 2, I-00185 Rome, Italy *European Synchrotron Radiation Facility (ESRF), BP 220, 38043 Grenoble cedex, France oDepartment of Physics, Western Michigan University, Kalamazoo, Michigan 49008, USA Metal-ammonia solutions are liquid systems that present many interesting properties. They are obtained by diluting alkali metals in liquid ammonia. Depending on the concentration of the alkali metals these compounds behave as insulators (where the electrical conduction is dominated by electrolytic mechanisms) or as liquid metals (where the electrical conduction is dominated by the free electrons response). They have been largely studied in the past [1] and are now the object of a renewed interest for two main reasons: their peculiar electronic behavior (e.g. see [2]) and their ionic arrangement (e.g. see [3,4]). In order to get information on both these aspects we performed static and dynamic structure factors measurements of lithium ammonia solutions by means of x-rays spectroscopy. The solutions were studied at several concentrations from the saturated metallic limit to the highly diluted non metallic region. The static structure factor study shows the progressive deviation of the lithium ammonia complexes spacing from the maxima of the Friedel oscillation governed by the static electronic response with the incoming of the metal non metal transition. While the dynamic structure factor study shows the increasing weight of the electronic correlations on the long-wavelength dynamics of these systems characterized by very low conducting electron densities. [1] J. C. Thompson, Electrons in Liquid Ammonia (Clarendon, Oxford, 1976); G. Lepoutre and P-J Lelieur, in MetalAmmonia Solution: proceedings of an international Conference on the Nature of Metal-Ammonia Solutions, Colloque Weyl II, edited by J.J. Lagowski and M. J. Sienko (Buterworths, London, 1970). [2] C.A. Burns et al., Phys. Rev. Lett. 86, 2357 (2001); Phys. Rev. Lett. 89, 236404 (2002). [3] S. Hayama et al., J. Chem. Phys. 116, 2991 (2002). [4] H. Thompson et al., J. Am. Chem. Soc. 125, 2572 (2003).