bip22654-sup-0001-suppinfo
advertisement

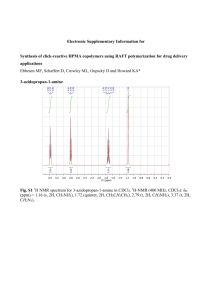
NMR data for small molecules acylated in solution phase Compound 107. 1H NMR (d6-DMSO, 400 MHz): δH 1.84 (3H, s), 3.72 (2H, d, J = 5.6 Hz), 8.15 (1H, bs), 12.48 (1H, bs); 13C NMR (d6-DMSO, 100 MHz): δC 22.7, 39.2, 170.1, 171.8. Compound 108. 1H NMR (d6-DMSO, 400 MHz): δH 1.43-1.72 (5H, m), 1.92 (1H, m), 1.93 (3H, s), 3.18 (2H, m), 4.14-4.37 (4H, m), 7.30-7.80 (8H, m); 13 C NMR (d6-DMSO, 100 MHz): δC 13.3, 15.1, 20.6, 21.1, 31.0, 40.1, 46.1, 59.7, 111.7, 117.0, 117.1, 118.8, 118.9, 119.5, 133.3, 135.9, 136.1, 149.5, 164.1, 166.7. Compound 109. 1H NMR (d6-DMSO, 400 MHz): δH 1.82 (3H, s), 2.98-3.03 (1H, m), 3.15-3.20 (1H, m), 4.48 (1H, d, J = 5.2 Hz), 6.99 (1H, m), 7.07 (1H, m), 7.35 (1H, d, J = 8 Hz), 7.54 (1H, d, J = 8 Hz), 8.15 (1H, d, J = 8 Hz), 10.83 (1H, s), 12.6 (1H, bs); 13 C NMR (d6-DMSO, 100 MHz): δC 22.8, 27.6, 53.4, 110.4, 111.8, 118.6, 118.8, 121.3, 123.9, 127.6, 136.5, 169.7, 174.0. Compound 110. 1H NMR (d6-DMSO, 400 MHz): δH 1.88 (3H, s), 2.40 (3H, s), 7.44-7.80 (3H, m), 8.60 (1H, s); 13C NMR (d6-DMSO, 100 MHz): δC 23.2, 117.0, 130.7, 137.8, 168.3. Compound 111. 1H NMR (CDCl3, 400 MHz): δH 2.63 (3H, s), 7.04 (1H, d, J = 14.8 Hz), 7.68 (1H, s), 8.39 (1H, s); 13C NMR (CDCl3, 100 MHz): δC 23.2, 117.0, 130.7, 137.8, 168.3. Compound 112. 1H NMR (d6-DMSO, 400 MHz): δH 1.90 (3H, s), 3.07-4.67 (7H, m), 4.77 (1H, d, J = 4.4 Hz), 4.61 (1H, d, J = 3.6 Hz), 6.55 (1H, d, J = 6.0 Hz), 7.14 (1H, d, J = 9.2 Hz), 7.42 (1H, d, J = 8 Hz); 13C NMR (d6-DMSO, 100 MHz): δC 23.4, 54.4, 61.7, 67.5, 70.0, 77.9, 93.9, 171.1. Compound 113. 1H NMR (d6-DMSO, 400 MHz): δH 1.78 (3H, s), 2.86-2.92 (1H, m), 3.08-3.13 (1H, m), 4.44-4.49 (1H, m), 6.99-7.07 (2H, m), 7.32 (1H, d, J = 8 Hz), 7.41 (1H, s), 7.60 (1H, d, J = 8 Hz), 7.95 (1H, d, J = 8 Hz), 10.78 (1H, bs); 13C NMR (d6-DMSO, 100 MHz): δC 23.0, 28.2, 53.6, 110.9, 111.7, 118.6, 118.9, 121.2, 123.8, 127.8, 136.5, 169.4, 174.1. 1 14.8 K cal/mol 11.1 0.0 2.9 0.0 - 4.0 Figure S1. The energy profile diagram of the acylation reactions with phenyl malonic acid (red) and with benzyl malonic acid (blue). OH H2C C O O O Br Br 95 95a NH2 Br NH 96a O S NH H2C C O O NH2 100 Br 105 H 95b NH O 96 O OH B: H2C C O -H2O O -H2O OH 106 96b O S NH O NH 100a O O O S 100b NH NH OH N O O S -H2O NH N 110 Figure S2. The proposed mechanism for the formation of rearranged products from the ketene intermediate. 2