A- Anihistamines L..
advertisement

Dr. Nadia Haress
INTRODUCTION
This course describes the medicinal chemistry of an important class of
drugs that act on the CNS. The therapeutic agents that are used to treat many of
the CNS-based disorders shall be studied, such as, antihistaminics, CNS
stimulants, sedatives, hypnotics, antidepressants, analgesics and general
anesthetics.
In each class of drugs you must know:
1. Structures.
2. Nomenclature.
3. Mode or mechanism of action.
4. Uses.
5. Structure activity relationships (SAR).
6. Synthesis.
7. Metabolism.
1
Histamine and Antihistaminics
Introduction
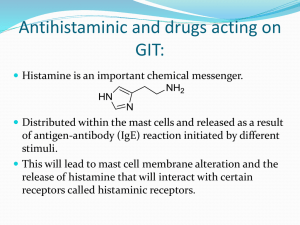
Histamine is a physiologically active, endogenous substance (autacoid)
that is produced within the body by decarboxylation of the amino acid histidine
and then stored in mast cells and basophils.
Chemistry
Histamine, known as [2-(imidazol-4-yl)ethylamine], consists of an
imidazole heterocycle and ethylamine side chain.
Histamine is a basic organic compound which has pKa values of 9.4
(aliphatic primary amine) and 5.8 (imidazole). Thus, it exists as an
equilibrium mixture of tautomeric cations at physiologic pH (7.4) with the
monocation dominating (about 96%) over the dication (about 3%) and
the nonprotonated species (about 1%).
NH3
N
NH3
+
NH
H3O
HN
+
NH
pKa 5.80
pKa 9.40
4
5
HN
1
NH2
NH2
N3
N
NH
2
Tautomers of histamine
2
H2O
Chemical Properties of Histamine
1) Imidazole N at position 3 N(π)→ near side chain.
2) Imidazole N at position 1 N (τ) far side chain.
3) The side chain N -NH2.
Histamine has been found to exist almost exclusively as a monocationic
conjugate species (-NH+).
The ratio of concentrations of tautomers
Nτ-H/Nπ-H has been calculated to be 4.2 indicating that in aqueous
solution 80% of histamine monocation exists as Nτ-H and 20% as Nπ-H.
Structure-activity relationship (SAR) studies suggest that -NH+
monocation is important for agonist activity at histamine receptors and
that the transient existence of the more lipophilic uncharged
(unprotonated) histamine species may contribute to the translocation or
transfer across the cell membranes. Other studies support that Nτ-H
tautomer of histamine monocation is the pharmacophoric species at the
H1-receptor whereas a 1,3-tautomeric system is important for selective
H2-receptor agonist.
Histamine Receptors
Once released, the physiological effects of histamine are mediated by cellsurface receptors. Extensive pharmacological analysis suggests the existence of
four different histamine-receptors subtypes, H1, H2, H3 and H4.
3
Histamine H1-receptors: Mediate smooth muscle contraction (GIT,
bronchi and uterus) , relaxation of blood vessels capillaries (ventricular
diseases) and pruritus.
H2-receptors: are located on the cell membrane of acid secreting cells of
the gastric mucosa and mediate the gastric acid secretory actions of
histamine (↑ production of gastric acid).
H3 –receptors:
found in the CNS; serving to modulate histamine
synthesis and release in the CNS(alertness, feeding, drinking…).
H4-receptors: located in bone marrow and white blood cells. They are
associated with chemotactic and inflammatory responses.
Actions of Histamine
1- Important allergic (chemical) mediator of hypersensitivity reactions.
2- Increases gastric acid secretion.
3- Neurotransmitter (stimulatory) in CNS.
Metabolism
Once released, histamine is rapidly metabolized in vivo to inactive
metabolites via enzymatic inactivation.
Uses
Histamine has no therapeutic application.
4
It is used as a diagnostic agent to test the secretory action of the stomach,
diagnosis of pheochromocytoma and as a positive control in the allergy
skin testing.
Antihistaminics
(Anti-Allergic Drugs)
Antihistamines (antihistaminics) are drugs which inhibit the actions of
histamine by competitively blocking the histamine receptors.
A)
Histamine H1-Receptor Antagonists:
They competitively inhibit the action of histamine on the tissues
containing H1-receptors.
I.
First-Generation H1-Antihistamines: (Classical
Antihistamines)
Structure-Activity Relationship (SAR):
R
Ar
X (CH2)n N
R-
Ar-
General Antihistamine Structure
5
a) Ar is aryl e.g., phenyl, substituted phenyl and heteroaryl, such as,
2-pyridyl. While Ar' is a second aryl or aryl methyl group. These two
aromatic systetems may be linked as in the tricyclic antihistamines
(phenothiazines, dibenzocycloheptanes and heptenes). The two aryl
moieties must be capable of adopting a non-coplanar conformation
relative to each other for optimal interaction with H1-receptor.
b) X is a connecting atom, which may be a saturated carbon-oxygen moiety as
in the aminoalkyl ethers, nitrogen atom as in case of ethylenediamine or a
carbon atom as in the propylamine antihistamines.
c) The carbon chain of a typical H1-antagonist (blocker) consists of 2 or 3
carbon atoms resulting in a distance of 5 to 6 Å between the centre point
of the diaryl ring system and the terminal nitrogen.
d) Substitution in the p-position of Ar or Ar’ by a halogen atom (Cl or Br)
enhances the antihistaminic activity.
e) In compounds that exhibit optical and geometrical isomerism, the
dextro(d)-isomers (S-configuration) are more active and the transisomers (pheniramine and chlorpheniramine) are more active as H1histamine receptors ( stereoselective).
f) The first (classical) and second (nonsedating) generation antihistamines
are more lipophilic than endogenous agonist histamine (or the H2antagonists). This lipophilicity difference results from the presence of the
2 aryl rings and the substituted amino moieties.
1)
Aminoalkyl Ethers (Ethanolamines)
6
R
R
Ar-
O CH2 CH2 N
R-
Ar
They are characterized by having CHO group as the connecting moiety (X)
and a 2 or 3 carbon atom chain as the linking moiety between the diaryl and
tertiary amino group.
Diphenhydramine (Benadryl)
2-(Diphenylmethoxy)-N,N-dimethylethanamine
It is used as a potent antihistamine that possess antitussive and sedative
properties.
Uses:
Used in the treatment of urticaria, seasonal rhinitis and some dermatoses.
7
Synthesis:
Dimenhydrinate (Dramamine)
8-Chlorotheophylline-2-(diphenylmethoxy)-N,N-dimethylethylamine
Uses
Widely used in the treatment of motion sickness.
Carbinoxamine (Colistin)
CH3
H
Cl
C O
N
CH3
N
2-{p-Chloro-[2-(dimethylamino)ethoxy]benzyl}pyridine
8
Carbinoxamine is a potent antihistaminic and is available as the racemic
mixture.
2)
Ethylenediamines
Ar
R
N CH2 CH2 N
Ar-
R-
They are characterized by having a nitrogen connecting atom (X) and a
two-carbon chain as the linking moiety between the diaryl and tertiary amino
moieties.
Tripelennamine (Pyribenzamine)
CH2 NCH2CH2N(CH3)2
N
2-{Benzyl[2-(dimethylamino)ethyl]amino}pyridine
Uses:
It is effective as diphenhydramine with less side effects.
3)
Cyclic Basic Chain Analogues
These compounds represent molecular modification of the general
ethylenediamine structure in which the basic dimethylamino group is replaced
by a small basic heterocyclic ring. These structures contain the same structural
elements as those of simpler ethylenediamines.
9
Clemizole
{1-[(4-Chlorophenyl)methyl]-2-(1-pyrrolidinylmethyl)-1H-benzimidazole}
Antazoline
[4,5-Dihydro-N-phenyl-N-(phenylmethyl)-1H-imidazole-2-methanamine]
Thenaldine
[1-Methyl-N-phenyl-N-(2-thienylmethyl)-4-piperidinamine]
4)
Monoaminopropyl Analogues (Propylamines)
10
R
Ar
CH CH2 CH2
Ar-
N
R-
The propylamines are characterized by having a carbon atom connected
with a carbon chain of 2 additional carbons linking the key tertiary amino and
diaryl pharmacophore moieties. They are commonly known as pheniramines.
Pheniramine Maleate
Chlorpheniramine Maleate
Brompheniramine Maleate
R
H
Cl
Br
Pheniramine maleate
Chlorpheniramine maleate
Brompheniramine maleate
Insertion of a halogen into the p-position of the phenyl ring as in
chlorpheniramine and brompheniramine increases potency twenty-fold and the
dextrorotatory isomers exhibit greater potency i.e., stereoselective H1-receptor
antagonist activity is observed with these compounds. They are more potent and
have longer duration of action.
11
Other important compounds in this series may be defined as unsaturated
(olefinic) analogue of monoaminopropyl antihistamines.
Pyrrobutamine Phosphate
1-[4-(4-Chlorophenyl)-3-phenyl-2-butenyl] pyrrolidine
It is long acting with comparatively slow onset of action.
Triprolidine Hydrochloride
(E)-2-[1-(4-methylphenyhl)-3-(1-pyrrolidinyl)-1-propenyl]pyridine
It is the most active as H1 antagonist. The activity is confined mainly to
the geometric isomer in which the phenylmethyl group is trans to the 2-pyridyl
group.
12
5)
Piperazine Derivatives
Cyclizine (Marezine)
1-Diphenylmethyl-4-methylpiperazine
Chlorcyclizine
Meclizine
Buclizine
1-(p-tert-Butylbenzyl)-4-(p-chlorophenylbenzyl)piperazine
All of the piperazine derivatives show:
Potent antihistaminic activity.
13
6)
CNS depressant action (↑ lipid solubility).
Slow onset and prolonged duration of action.
Antiemetic activity (Buclizine and Meclizine), given during pregnancy.
Tricyclic Ring System (Phenothiazines and
Dicycloheptenes)
6
7
5
S
4
3
10
8
9
N
R
2
1
In addition to H1 antagonist activity these compounds have tranquilizing
and antiemetic activities; they potentiate the effect of analgesics and sedatives.
Promethazine Hydrochloride
10-[3-(Dimethylamino)-2-methylpropyl]phenothiazine
In addition to its antihistaminic action, it is a potent antiemetic and
sedating agent.
14
Methdilazine Hydrochloride
10-[(1-Methyl-3-pyrrolidinyl)methyl]phenothiazine
Isothipendyl Hydrochloride
The nitrogen-containing isostere of phenothiazine 1-azaphenothiazine,
was used successfully in the preparation of isothipendyl HCl.
The nitrogen atom in the phenothiazine ring system may be replaced by
an sp2 or sp3 carbon atom resulting in tricyclic thioxanthene series without loss
of activity.
Pimethixene
[1-Methyl-3-(9H-thioxanthen-9-ylidene)piperidine]
15
A potent H1-antagonist and it is an example of a thioxanthene compound
with an sp2 carbon atom.
Cyproheptadine Hydrochloride (Periactin)
It is also related to the phenothiazines where the S in the tricyclic ring is
replaced by –CH=CH- (an example of bioisosterism) i.e., S and –CH=CH- are
similar in size and similar biological activities are observed.
It exhibits both H1 antagonist and antiserotonin activity.
Azatadine
6,11-Dihydro-11-(1-methyl-4-piperidinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine.
It is the most potent analogue in this series. It is a potent long acting
antihistaminic with antiserotonin activity.
16
Ketotifen (Zaditen)
4,9-Dihydro-4-(1-methyl-4-piperidinylidene)-10H-benzo[4,5]cyclohepta[1,2-b]thiopehen-10-one.
It is an interesting compound as it is a potent H1 antagonist and is also
used in the treatment of recurrent vascular headache including migraine. In
addition, it is used in the prophylactic treatment of asthma.
17
II.
Second-Generation H1-Antihistamines (Nonsedating
Antihistaminics)
The second-generation antihistamines are more similar pharmacologically than structurally. They were developed as selective H1-receptor
antagonists with relatively high potency and decreased sedative effects (
penetration to BBB).
Most of these compounds produce prolonged antihistaminic effect as a
result of slow dissociation from H1-receptors.
Most of these drugs are administered once daily.
Terfenadine
-[4-(1,1-Dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1piperidinebutanol
It is an H1-antagnist with no CNS side effects (sedation, fatigue and
dizziness); but makes dangerous cardiac arrhythmias.
Fexofenadine (Allegra)
18
It is the carboxylic acid metabolite of terfenadine. It accounts for the
antihistaminic properties of terfenadine but it is less potent than it.
Ebastine
Ebastine is a potent selective H1 antihistamine. Its pharmacologically
active
acid
metabolite,
Carebastine,
is
metabolically
similar
to
fexofenadine(oxidation of the tertiary butyl group),the acid metabolite of
terfenadine.
Carebastine is more potent than its parent compound ebastine.
Citrizine
It is the main acid metabolite of hydroxyzine, highly polar, so it does not
penetrate the blood brain barrier (BBB) and so it has reduced sedative effects.
19
Loratadine (Claritin)
Loratadine is hydrophobic analogue of azatadine.
It is a potent H1-antagonist with no cardiotoxic effects.
Desloratadine (Clarinex)
Cl
N
N
H
Desloratadine is the active metabolite of loratadine (descarboethoxyloratadine).
It is equipotent as loratadine with no cardiotoxic effects.
It is used also as a decongestant.
20
III.
Inhibition of Histamine Release (Mast Cell Stabilizers)
The bronchiodilatory activity of khellin (a chromone obtained from a
plant known as Ammi visnaga) used by ancient Egyptians for spasmolytic
activity,
stimulated
the
search
for
related
compounds
with
similar
pharmacological properties for the treatment of hypersensitivity (Bis
chromones).
Cromolyn Sodium (Intal)
It inhibits the release of histamine, leukotrienes and other substances
from mast cells during allergic response.
Cromolyn is used prophylactically for bronchial asthma (inhaled as
powder) and for prevention of exercise induced bronchospasm.
21
Nedocromil Sodium
Disodium 9-ethyl-6,9-dihydro-4,6-dioxo-10-propyl-4H-pyrano[3,2-g]quinoline2,8-dicarboxylate.
Unlike the relationship of the aryl rings in H1-antagonists, coplanarity of
the two chromone rings is the most important requirement for biological
activity in the chromone series.
This drug is also administered by inhalation for the prophylactic
treatment of asthma.
22
B)
Histamine H2-Receptor Antagonists (Antiulcer Agents)
1-
H2-Receptor Antagonistic Drugs
These drugs competitively inhibit the action of histamine at H 2-receptors.
The H2-antihistamines are used in the treatment of duodenal ulcers, gastric
ulcers and gastroesophageal reflux disease (GERD).
Structure-Activity Relationships (SAR)
a) The imidazole ring of histamine is not required for competitive
antagonism of histamine at H2-receptor. Other heterocyclic rings may be
used. However, if the imidazole ring is used, the Nτ-H tautomer should be
predominant for maximal H2-antagonistic activity.
b) Separation of the ring and the nitrogen group – R’ (terminal nitrogen)
with a four carbon chain appears to be necessary for optimal activity.
c) The terminal nitrogen should be polar and nonbasic for maximal binding
to the receptor.
23
Burimamide
The structure of burimamide favours the Nπ-H tautomer (less favourable
for H2-binding).
Burimamide is not useful clinically as the thiourea moiety decreases the
antagonistic activity and causes side effects e.g. agranulocytosis.
Metiamide
Substitution of one methylene (CH2) group in the side chain of
burimamide with an isosteric sulpher atom and the addition of one
methyl group to position 5 on the imidazole ring resulted in another agent
(metiamide).
The dominating structure is the Nτ-H tautomer (favourable for H2receptor binding).
It was also not marketed because of the similar side effects
(agranulocytosis) as in burimamide caused by the thiourea function.
24
Cimetidine (Tagamet)
N-cyano-N’-methyl-N"-[2[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl]guanidine
Interestingly, the cyanoguanidine functionality is isoelectronic with the
thiourea group. So this substitution results in cimetidine which is devoid
of the undesirable side effects possessed by metiamide and related
thioureas.
Cimetidine is used for treatment of conditions associated with gastric
hyperacidity.
Synthesis:
25
It is synthesized from the reaction of 4,5-disubstituted imidazoles and
-mercaptoethylamine HCl, generating -imidazolyl (methylthio) intermediate,
which on condensation with N-cyano-N'-S-dimethylisothiourea, generates the
desired compound.
Several second-generation H2 antagonists, introduced in 1980s, use
aromatic and other heteroaromatic groups in place of the imidazole
functionality. These agents include ranitidine, nizatidine and famotidine.
Ranitidine (Zantac)
It is an aminoalkyl furan.
It is 4-10 times more potent than cimetidine.
Replacement of the furan ring by a thiazole group generates nizatidine.
26
Nizatidine
It is 5-18 times more potent than cimetidine.
Famotidine
It
is
a
histamine
H2-receptor
antagonist
where
the
basic
dimethylaminomethyl group is replaced by the guanidine functionality.
2-
Antiulcer Agents Acting by Other Mechanisms Other Than
H2-Receptor Blockade)
i)
Proton Pump Inhibitors (PPI)
The final step in acid secretion in parietal cells of the gastric mucosa is
mediated by H+/K+-ATPase (the gastric proton pump).
Mode of Action:
Omeprazole, lansoprazole and related analogues, produce irreversible
inhibition of gastric acid secretion by:
27
1) Rearrangement in the strongly acidic environment of the parietal
cells.
2) Then covalent binding of the rearranged inhibitor (drug) by
disulphide bond to the active site of cysteine rich H+/K+-ATPase
resulting in inactivation of the catalytic function of the proton
pump.
Omeprazole (Losec)
5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulphinyl]-1Hbenzimidazole
28
Figure: Acid-catalyzed activation of omeprazole to reactive sulfonamide. At the
parietal cell, H+/K+-ATPase, a cysteine residue, reacts to form disulfide-attached
enzyme inhibitor.
Because the initial rearrangement only occurs at a strongly acidic pH,
acid-stable oral dosage forms are used that allow dissolution, release and
absorption of the drug in the duodenum.
So, the drug must be taken as enteric-coated granules in capsules or
enteric coated tablets.
ii)
Prostaglandins
Prostaglandins have antisecretory effects on gastric acid. Also they
stimulate secretion of mucous.
29
Misoprostol
Misoprostol is a synthetic analogue of prostaglandin E1 (PGE1).
It is used mainly in the treatment of duodenal ulcers which are
unresponsive to H2-antagonists.
iii)
Chemical Complexation
The sulphate esters and sulphonate derivatives of polysaccharides and
lignin form chemical complexes with enzyme pepsin.
Sucralfate
Sucralfate is a complex of the sulphuric acid ester of sucrose and
aluminium hydroxide.
30
This physical complex binds or adheres to the surface of the crater of the
ulcerated area in the stomach, protecting the ulcer from erosive action of
pepsin.
C)
H3-Receptor Antagonists
The histamine H3-receptors play a role as a general regulatory receptor
system, modulating not only the release and synthesis of but also the release of
other neurotransmitters. These antagonists were discovered within a group of
H2-receptor antagonists. Burimamide, the first H2-receptor antagonist, is 100fold more potent at H3 than at H2-receptors.
Thioperamide
It is a drug of high affinity and selective H3-receptor antagonist.
It may be useful in treatment of allergic rhinitis but mainly effective in
animal models.
31
Imetit
S-[(2-imidazol-4-yl)ethyl]isothiourea
It is a potent and selective H3-agonist.
It is used mainly as pharmacological tool (animal models).
D)
H4-Receptor Antagonists
The H4-receptor is expressed in mast cells, eosinophils and basophils. It is
a relatively recent discovery; thus, much is not yet understood about its
physiological role.
It is thought to play a role in inflammatory responses, especially in the
mediation of chemotactic responses.
A selective H4-antihistamine has been recently reported, JNJ 777120.
It blocks mediated inflammatory responses.
Other conditions in which H4-antagonists might be useful include
autoimmune inflammatory and allergic disorders, including rheumatoid
arthritis, asthma and allergic rhinitis.
32