projWork (boni)
advertisement

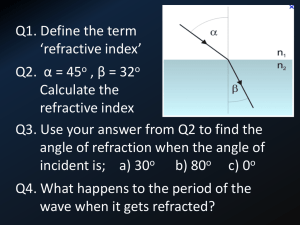
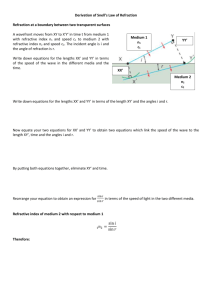
REFRACTIVE INDEX OF SALT SOLUTION AS RELATED TO ITS SALINITY AND CONCENTRATION by: Caculitan, Bonifacio Jr. D. LEAP-CTP1 National Institute of Education, Singapore October 2009 REFRACTIVE INDEX OF SALT SOLUTION AS RELATED TO ITS SALINITY AND CONCENTRATION ______________________________ A project work presented to A/P Augustine Tan Tuck Tee In partial fulfillment of the Requirements in Physics Pedagogical Knowledge ______________________________ by: Caculitan, Bonifacio Jr. D. LEAP-CTP1 National Institute of Education, Singapore October 2009 REFRACTIVE INDEX OF SALT SOLUTION AS RELATED TO ITS SALINITY AND CONCENTRATION Caculitan, Bonifacio Jr. D. LEAP-CTP1 National Institute of Education, Singapore ABSTRACT A study on refractive index of salt solution was conducted to determine its effect to salinity and concentration. Specifically the study aimed to apply Snell’s Law in determining the refractive indices of salt solutions at different salinity, and concentration at constant temperature. The following hypotheses were tested in this study: 1) There is no difference with the computed value of refractive index at different salinity and concentration at constant temperature; and 2) Concentration and salinity of salt solution do not affect refractive index. Results showed that to obtain the standard value of refractive index, salinity, and concentration for water used in aquaria, at 40 degrees angle of incidence angle of refraction must be 59.50 degrees. The results also showed that as refractive index of salt solution increases, salinity and concentration also increases. Based on the result of the study, the null hypotheses were rejected. It is finally recommended that salinity can be obtained using Snell’s law. Aquarists can easily maintain the salinity of water in their aquarium by simply observing the angles of incidence and refraction without necessarily obtaining the value of refractive index. INTRODUCTION Snell's law (also known as Descartes' law, the Snell–Descartes law, and the law of refraction), is a formula used to describe the relationship between the angles of incidence and refraction, when referring to light or other waves passing through a boundary between two different isotropic media, such as glass, water, and salt solution. The law says that the ratio of the sines of the angles of incidence and refraction is a constant that depends on the media. Named after Dutch mathematician Willebrord Snellius, Snell's law states that the ratio of the sines of the angles of incidence and refraction is equivalent to the ratio of velocities in the two media, or equivalent to the opposite ratio of the indices of refraction. In optics, the law is used in ray tracing to compute the angles of incidence or refraction, and in experimental optics to find the refractive index of a material. Refractive index is one of the most important optical properties of a medium. It plays vital role in many areas of physical science with special reference to fiber optics. Similarly, measurement of refractive index is widely used in analytical chemistry to determine the concentration of solutions. Some studies (Schwartz 1999, Olesberg 2000, Shlichta 1986) provide more detailed discussion on the concentration mapping by the measurement of refractive index of liquids. Several techniques are reported in literature for the measurement of concentration dependence of refractive index of liquids (McPherson 1999, Garcia 1999, Otalora 1999, Miyashita 1994). The present study uses a relatively simple and effective technique, which can be used to measure the refractive index of salt solution at different concentration. On the other hand, according to Holmes-Farley (Reefkeeping Magazine, Volume 8, No. 3 April 2009 Issue), salinity is one of the most important parameters measured in reef aquaria. It controls not only the salt balance between an organism and its surrounding environment, but also the levels of a host of ions in seawater that aquarists neither measure nor control independently. Consequently, aquarists must monitor salinity to ensure that organisms are not stressed by moving between aquaria of potentially different salinity, and that the salinity of the aquarium itself is controlled within ranges that organisms thrive in. Seawater is approximately 96.5% water and 3.5% salt, by weight. When the salinity of seawater is referred to as being 35 ppt (parts per thousand), that is the same as saying 3.5% salt. Seawater is composed of many different ions (salts), with the total of all adding up to the 3.5%. Some ions are present at very high concentration and some at very low concentration. However, even those present at very low concentration can be important to organisms living in the water. When salts are dissolved in water, a variety of the water's attributes change, including its density, its refractive index and its conductivity. All of these changes can be used by aquarists as a way to measure the total amount of salt in solution. Consequently, aquarists need not dry out water to find the salinity by the amounts of salts that remain behind, but can simply rely on tools that measure these other attributes. Hence, salinity is considered in this study to determine its relation to refractive index. Objectives: Specifically, the study aimed to: 1. Apply Snell’s Law in determining the refractive indices of salt solutions at different salinity, and concentration at constant temperature; 2. Determine the effect of refractive index to concentration and salinity of salt solutions. Hypotheses: The following hypotheses were tested in this study: 1. There is no difference with the computed value of refractive index at different salinity and concentration at constant temperature. 2. Concentration and salinity do not affect refractive index of salt solution. Significance of the Study The study is significant to the aquarists who need to maintain the salinity of their aquarium by just considering the angles of incidence and refraction to come up with the refractive index. Likewise the study is significant to students and researchers who are trying to determine the effect of refractive index and salinity of salt solution. METHODOLOGY Materials and Equipment The following materials and equipment were used in this study: Laser Refraction tank is used to measure the angle of incidence and angle of refraction to come up with refractive index. Beaker is used to measure the volume of purified water. Digital Weighing Balance is used to measure the mass of purified water and iodized salt. Teaspoon is used to transfer iodized from container to the solution container. Laboratory thermometer is used to measure the temperature of the solution. Purified water is used as solvent for the solution. Iodized salt is used as solute for the solution. PROCEDURE The flow chart shows the stages of the procedure employed in this study: Preparation and gathering of materials Measuring the volume and mass of purified water that will be accommodated in the laser refraction tank Measuring the angles of incidence (40 O) and refraction of purified water using the laser refraction tank Recording and analyzing the results Computing for the refractive index using the equation, 𝑟 𝑛 = 𝑆𝑖𝑛𝑒 𝑖 Measuring the angles of incidence (40 O) and refraction of salt solution using the laser refraction tank Recording the concentration and salinity of salt solution Computing for the refractive index using the equation, 𝑟 𝑛 = 𝑆𝑖𝑛𝑒 𝑖 Measuring the temperature of purified water accommodated in the laser refraction tank Preparing the salt solution at different concentration Weighing the amount of purified water and iodized salt for the solution Preparation and Gathering of materials. Before starting the experiment, see to it that all the materials needed are already prepared. In the absence of purified water, tapped water can be a good substitute. Table salt may be used in the absence of iodized salt. Measuring the volume and mass of water. It is also necessary to note the amount of water just enough to fill one-half the laser refraction tank. Measure the mass of this volume and record it. This amount is necessary in preparation of the salt solution. Measuring the angles of incidence and refraction of water. Using the laser refraction tank, set the angle of incidence at 40 degrees (40O ) then record the resulting angle of refraction. Computing for refractive index. Compute for refractive index, using the equation, n=Sin r /Sin i where: n – refractive index i – Angle of incidence r – Angle of refraction Measuring and recording the temperature. It is necessary to measure and record the temperature of the water or solution because temperature might affect the result of the experiment. Weighing the amount of water and salt. The volume of water accommodated in the laser refraction tank is considered to determine the amount of water and salt that will be mixed into a solution. For example, in 1000 ml of seawater, 96.5% of it is water and 3.5% is salt by mass. By obtaining 96.5%, there must be 965 grams of water and 3.5% or 35 grams of salt. Preparation of salt solution at different concentration. In preparing the salt solution, start with 0.2% of salt and 99.8% water, then 0.4% salt and 99.6% water, then 0.6% salt and 99.4% water and so on. Recording the concentration and salinity of the solution. Record the concentration and salinity of the solution on a table. For the concentration, use % and grams .For the salinity use parts per thousand (ppt). Measuring the angles of incidence and refraction of the salt solution. Using the laser refraction tank, set the angle of incidence at 40 degrees (40 O ) then record the resulting angle of refraction in all different concentration of salt solution . Computing for refractive indices of salt solution at different concentration. Compute for refractive index, using the equation, n=Sin r /Sin i where: n – refractive index i – Angle of incidence r – Angle of refraction Recording and analyzing the results. Record and analyze the results properly. RESULTS AND DISCUSSION Refractive Index of Salt Solution at Different Salinity and Concentration Concentration Water (ml) of salt(g) 0.00 350.00 Salinity (ppt) 0 Angle of Incidence (o) 40 Angle of Refractive o Refraction ( ) Index 59.00 1.333 3.50 346.50 10 40 59.25 1.336 5.25 344.75 15 40 59.25 1.336 7.00 343.00 20 40 59.25 1.336 8.75 341.25 25 40 59.25 1.336 10.50 339.50 30 40 59.25 1.336 12.25 337.75 35 40 59.50 1.339 14.00 336.00 40 40 59.50 1.339 15.75 334.25 45 40 59.50 1.339 17.50 332.50 50 40 59.75 1.342 19.25 330.75 55 40 59.75 1.342 21.00 329.00 60 40 59.75 1.342 22.75 327.25 65 40 59.75 1.342 24.50 325.50 70 40 60.00 1.347 26.25 323.75 75 40 60.00 1.347 28.00 322.00 80 40 60.00 1.347 Table 1 shows the refractive indices of water and salt solution at different concentration and salinity. As shown in the table, column 1 is the concentration of salt in grams, column 2 is the amount of water in milliliter (ml), column 3 is the salinity of salt solution in parts per thousand (ppt), column 4 is the angle of incidence in degrees, column 5 is the angle of refraction in degrees, and the last column is the refractive index. It showed in column 3 that the angle of incidence was controlled at 4o degrees. The zero value (row 2) on the concentration means that no amount of salt was added, meaning the liquid used was pure water. At 40 degrees, the resulting angle of refraction in pure water is 59 degrees. Applying Snell’s law, the computed value of the water is 1.333 which is the same as the standard refractive index of water which is 1.333. When salt was added, from row 3 to 11, the obtained value of refractive index differs. It showed that as more salt was added to the solution to increase salinity and concentration, the greater is the obtained value of refractive index. Furthermore, the yellow rows show the standard, seawater in the open ocean has a salinity of 1.339 as stated by Michael J. Kennish (2000) in his book “Practical Handbook of Marine Science”. The obtained value of refractive index in this experiment is 1.339 which is also the standard value. The results show that at 40 degrees as angle of incidence, the angle of refraction must be 59.50 to come up with the standard value of salinity. Aquarists therefore can maintain the salinity of water in their aquaria by simply measuring the angle of incidence at 40 degrees and the angle of refraction must be close to 59.5 degrees. Hence the results show varied values of obtained refractive index, the null hypothesis that there is no difference on the computed value of refractive index at different concentration and salinity is rejected. Refractive Index Refractive Index vs Concentration Graph 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 2 4 6 8 10 12 14 Concentration (g) 16 18 20 Figure 1. Refractive Index vs Concentration Graph Figure 1 shows the refractive index against concentration line graph. In this graph 100 ml of water was used but the same concentration was maintained, this was done for convenience of measuring the amount of salt. As shown by the slight inclination of the line there is a slight increase of refractive index as the concentration gradually increases. The obtained value of refractive index ranges between 1.333 to 1.447 which means that concentration of salt solution affects refractive index. The gradual inclination of the line shows that concentration affects refractive index, thus, the null hypothesis that concentration does not affect refractive index is rejected. Refractive Index vs Salinity Graph 1.5 1.4 1.3 1.2 1.1 Refractive Index 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 Salinity (ppt) Figure 2. Refractive Index vs Salinity Graph Figure 2 shows the refractive index against salinity graph. The straight line shows the gradual increase of refractive index as salinity increases. Refractive index ranges from 1.333 to 1.347 while the salinity ranges from 0 to 75 parts per thousand (ppt). The slight inclination of the line shows that salinity affects refractive index. The null hypothesis that salinity does not affect refractive index is rejected. CONCLUSION AND RECOMMENDATIONS Conclusion Based on the results of the experiment the following conclusions are made: 1. Concentration and salinity affect refractive index of salt solution. Refractive index increases as concentration and salinity of salt solution increases. 2. At 40 degrees angle of incidence, the required angle of refraction must be 59.50 degrees in order to come up with the standard salinity of an aquarium. 3. Snell’s law can help aquarists in maintaining the salinity of water in their aquarium. Recommendations Based on the results of the experiment it is therefore recommended that: 1. Salinity can be obtained using Snell’s law. Aquarists can maintain the salinity of water in their aquarium by merely observing the angles of incidence and refraction without necessarily obtaining the value of refractive index. 2. The results of the experiment can be conveniently used by aquarists as their basis in maintaining salinity of water in their aquarium. 3. A parallel study must be conducted further to determine whether the results will be the same as the results of this study. BIBLIOGRAPHY REFERENCES 1. Garcia-Ruiz, J. M., Novella, M. L., Otalora, F., Supersaturation patterns in counter-diffusion crystallisation methods followed by Mach-Zehnder interferometry, Crystal Growth, (1999) 196, 703-710. 2. Holmes-Farley, Randy. Reefkeeping Magazine, Reef Central, LLC Copyright 2008. 3. Kennish Michael J. Practical Handbook of Marine Science, Third Edition (2000) 896 pp, Publisher: Lewis Publishers, Inc. 4. McPherson, A., Malkin, A. J., Kuznetsov, Y. G., Koszelak, S., Wells, M., Jenkins, G., Howard, J., Lawson, G., Effects of microgravity on protein crystallization: evidence for concentration gradients around growing crystals , J. Crystal Growth, (1999) 196, 572. 5. Miller, A., Hussmann, E. K., McLaughlin, W. L., Interferometer for measuring fast changes of refractive index and temperature in transparent liquids Review of Scientific instruments, (1985) 46, 1635. 6. Shlichta, P. J., Feasibility of mapping solution properties during the growth of protein crystals. J. Crystal Growth, (1986) 76, 656. ACKNOWLEDGEMENT The researcher would like to express his deepest gratitude to Associate Professor Augustine Tan for his untiring support and guidance during the conduct of this study. Likewise to National Institute of Education, Singapore and Ateneo de Manila University Philippines, family and friends who supported the researcher in their own simple way. Above all, to Almighty God for the wisdom and power. The researcher