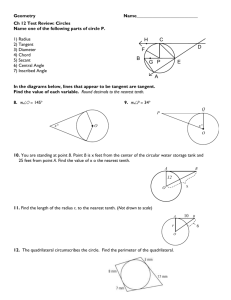
Name: Date: ______ Period
advertisement

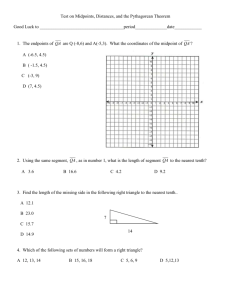
Name: ________________________________ Date: __________ Period: _______ Mathematical Quiz 1 for Cells (30 points) 1. The three cells below have the following dimensions. What is the surface to volume ratio, in lowest common denominator format, for each cell? (2 points/line) A) B) C) 3mm 3mm 1mm 2mm _____3__ _: ___1_____ 1mm 3mm ____2____ :____1_____ 1mm ______6___: ____1____ Which cell has the best has the best efficiency is transporting nutrients in and waste products out? (2 points) Why in 3 sentences or less? (6 points) ______Cube C is most efficient______ Explain: Mathematical Quiz 2 for Cells (30 points) 2. The three cells below have the following dimensions. What is the surface to volume ratio, in lowest common denominator format, for each cell? (2 points/line) A) B) C) 7 cm 5 cm __1.2_____ _: _1_______ 7 cm ___1_____ :____1.16_____ 10 cm 10 cm ___1______: ___1.7__ Which cell has the best has the best efficiency is transporting nutrients in and waste products out? (2 points) Why in 3 sentences or less? (6 points) Cube A is most efficient; explanation 3. An experiment to measure the rate of Hydrogen Peroxide decomposition by catalase at two different temperatures was performed and yielded the following data. Calculate the Q 10 to the nearest hundredth using the given data. You must show ALL of your work. (10 points) Temperature in ⁰C 25.5⁰C 35.5⁰C SHOW YOUR WORK HERE: 2.03 Decomposition reaction rate (kPa/Min.) 0.0315 0.0641 4. An experiment determined that when a digestive enzyme unfolds to its denatured (D) state from the original folded(F) state, the change in Entropy is ΔS = S(D) – S(F) = 154 joules/mol. The change in Enthalpy is ΔH = H(D) – H(F) = 53,500 joules/mol. At a temperature of 32⁰C, calculate the change in Free Energy ΔG, in j/mol, when the enzyme unfolds from its folded state. You must show all your work and circle your final answer. 6,530 j/mol 5. An experiment involving a species of photosynthetic Algae was conducted to try and measure the amount of carbon that was fixed into the composition of glucose molecules. The algae was placed in a one liter glass mason jar and then covered with the same pond water in which the algae was growing. The initial amount of dissolved oxygen (DO) in the water was determined to be 5.64 mg O2/L. The mason jar was then placed under a light source for 24 hours. The final reading for dissolved Oxygen was 11.83 mg O2/L. The mason jar remained at 6⁰C the whole time. Calculate the amount of carbon that was fixed, in photosynthesis, based on the change in amount of dissolved oxygen over the 24 hour period, using the formulas above. Show all your work below and circle your final answer. 2.315 mg C fixed/L 6. A bag of M&M was purchased for a science experiment on probability. The teacher counted 100 M&Ms. The data are listed below. Her hypothesis was: If there are four colors of M&Ms, there should be an equal number of each M& Ms for each color. Calculate X2 and determine if this experiment was successful. Color of M&M Brown Yellow Green Orange Observed (O) 22 24 29 25 X2 = 1.04 Df: 3 CV= 7.82 Accept Hypothesis bc X2 is less than the CV. 7. A science student at the University of Alabama Birmingham was performing a genetic mating experiment, using fruit flies, to test Mendel’s laws of inheritance and probability. The student counted 100 fruit flies total. The data are listed below. Her hypothesis was: If the color of white eyes is a recessive trait found on the X chromosome, there should be a predictable outcome of 1:1:1:1 in the F2 generation resulting from a carrier female and an affected male. Red eyes are the normal dominate color (also known as wild type) Calculate X2 and determine if this experiment was successful. Color of eye/sex Red/female White/female Red /male White/male Observed (O) 34 17 30 19 X2 = 8.24 Df = 3 Cv = 7.82 Reject hypothesis bc the X2 value is larger than the CV 8. Calculate the Total Water Potential for the following solution: The molar concentration of a sucrose solution in an open beaker has been determined to be 0.05M. The pressure potential of a solution open to the air is zero. What is the water potential for this example at 15 degrees C? Round your answer to the nearest tenth in bars. A bar is a water potential unit of measurement. Then circle your answer. -1.2 9. An experiment to measure the rate of Hydrogen Peroxide decomposition by catalase at two different temperatures was performed and yielded the following data. Calculate the Q 10 to the nearest hundredth using the given data. You must show ALL of your work. (10 points) Temperature in ⁰C 20.4⁰C 30.4⁰C 2.47 Decomposition reaction rate (kPa/Min.) 0.0289 0.0716 10. An experiment determined that when a lipid is oxidized in the process of cellular respiration within a cell, the change in Enthalpy is ΔH = 59.6 kJ/mol. Also the change in Entropy is ΔS = 210 joules/mol. At a temperature of 27⁰C, calculate(15 points) the change in Free Energy ΔG, in J/mol, and explain(5 points) if Free Energy is available for generating ATP. You must show all your work and circle your final answer. -3,400 j/mol 11. A genetics professor at Auburn University was having her freshmen Biology students perform a genetic mating experiment, using field mice, to test Mendel’s laws of inheritance and probability. The one group of students counted 60 mice total. The following genetic combinations produce the following hair types in mice. BB = Black; Bb = grey; bb= white. If the parent field mice were both Bb, what would be the expected outcome? Their hypothesis was, if two heterozygous parents are mated together, then the offspring should be roughly ¼ Black hairs, ½ Gray hairs, and ¼ white hairs. A 1:2:1 ratio. The data are listed below. Calculate X2 and determine if this experiment was successful. Color of hair BB - Black Bb - Grey bb- White Observed (O) 12 34 14 X2 = 1.2 Df = 2 CV = 5.99 Accept Hypothesis bc X2 is less than CV. 12. An experiment to measure the rate of protein decomposition by Carboxypeptidase, an enzyme in your body that helps you digest proteins, at two different temperatures was performed and yielded the following data. Calculate the Q 10 to the nearest hundredth using the given data. You must show ALL of your work. (10 points) Temperature in ⁰C 25.5⁰C 35.5⁰C Decomposition reaction rate (kPa/Min.) 0.0315 0.0933 2.96 13. The three cells below have the following dimensions. What is the volume for each cell? The height is indicated and so is the radius (r). Record each answer to the nearest tenth. (2 points/line) A) B) C) 6mm ______ r 3mm 169.6 mm3 4mm ______r 1mm 12.6 mm3 2mm ______r 2mm 25.1 mm3 Please use whole numbers to answer the following: How many times larger is the volume of Column A as compared to Column B? 13.5 How many times larger is the volume of Column C as compared to Column B? 2 14. A student is needing to make to make 1 liter of a 0.1 M solution of NaCl for a seed germination experiment. She weighs out 58.44 grams of NaCl and pours it into an Erlenmeyer flask and dilutes it to a total volume of 1 L. She now has 1 liter of a 1M solution. She wants to save her stock solution for making additional concentrations. How many mLs will she need to use to get her molar concentration down to a 0.1 M solution in a 1 liter volume? Show all your work and circle your final answer. Vi = 100 mL 15. The three cells below have the following radii (plural radius). Calculate the volume and Surface area for each cell? Then determine the Surface Area to Volume ratio for each sphere. Record all answers to the nearest tenth. (1 points/line) A) B) Radius 2mm V : 33.5 mm3 SA : 50.2 mm2 SA 1.5 : V 1 C) Radius 3mm V : 113.0 mm3 SA : 113.0mm2 SA 1 : V 1 Radius 4mm V : 268.0 mm3 SA : 201.0 mm2 SA 1 : V 1.3 Which sphere is most efficient at membrane transport of waste? (1 Point) A B C WHY? (3 points) ________________________________________________________________________ ________________________________________________________________________ ____________ Which sphere is least efficient at membrane transport of waste? (1Point) A B C WHY? (3 points) ________________________________________________________________________ ________________________________________________________________________ ____________ 16. The three flat pieces of metal below have the following dimensions. The height and length are given. What is the total square area for each piece? Record each answer to the nearest tenth. (2 points/line) Area = L X H A) 4.0 cm B) 6.5cm 7.0 cm 28 cm2 C) 12.3 cm 8.0 cm 52 cm2 5.6cm 68.9 cm2 17. The block of ice below has the following dimensions. The height, length, and width are given. Answer the following question with your answers rounded to the nearest hundreth. Be sure to show the proper units too. (2 points/line) Surface area of a rectangular solid 10 cm 6.5 cm 100 cm 3,430 cm 2 18. Parents: Male – AABBCC SHOW ALL YOUR WORK 1 or 100% Female – aabbcc DESIRED Offspring – AaBbCc 19. Parents: Male – AABbCc Female – AaBbCc DESIRED Offspring – AabbCC SHOW ALL YOUR WORK 0.031 or 3.1% 20. Parents: Female - AaBbCc SHOW ALL YOUR WORK 0.125 or 12.5% Male – AaBbCc DESIRED Offspring – AaBbCc 21. In the following graphic on population size over time, what is the rate of change between the sixth and tenth generation? 6000 (there could be other possible answers depending on how students read the Y axis data for the 6th and 10th generations. 22. A population of dung beetles in the African savannah exhibits logistical growth. The carrying capacity of the region is 500 beetles. The maximum per capita growth rate, r max, for the population is 0.2 dung beetles/month. Calculate the the maximum population growth rate for the population if the maximum occurs when N = K/2. Give your answer to the nearest tenth and circle it. 25.0 23. A population of fire ants in the Mobile Delta exhibits logistical growth. The carrying capacity of the ant mound is 15000 ants. The maximum per capita growth rate, rmax, for the population is 0.9 fire ants/month. Calculate the maximum population growth rate for the population if the maximum occurs when N = K/15. Give your answer to the nearest tenth and circle it. 840.0 24. A researcher working with walking stick insect are interested in the allelic frequency of a particular dominate allele with in the given population in north eastern Alabama. They estimate the percentage of the population, by the mark-recapture method, possessing the recessive trait to be at 16%. What is the frequency of the dominate allele within the population? 0.6