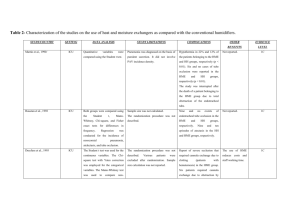
dln(q) / d(1/T g ) = -E a * /R Eq. 3
advertisement

Supplementary Material Molecular indicators of the surface and bulk instabilities of hot melt extrudated amorphous solid dispersions Ziyi Yang1,3, Kathrin Nollenberger 2, Jessica Albers 2, Duncan Craig3, Sheng Qi1* 1. School of Pharmacy, University of East Anglia, Norwich, Norfolk, UK, NR4 7TJ 2. Evonik Industries AG, Kirschenallee, Darmstadt, Germany 3. University College London, School of Pharmacy, London, UK,WC1N 1AX List of content: 1. Calculation of fragility index 2. Calculation of structural relaxation time 3. Calculation of interaction parameter and the prediction of drug-polymer solubility using melting point depression method 4. ATR-FTIR results of spin coated amorphous drugs aged under different conditions. 5. SEM images of the surface of 10% (w/w) HME felodipine-Eudragit® E PO and 10% HME celecoxib-Eudragit® E PO aged for 6 months under different conditions. 6. DSC results of 10% (w/w) HME samples after 6 months aging under different conditions. 1. Calculation of fragility index Fragility indexes of amorphous model drugs in this study were calculated by using the theory of extrapolating configurational entropy to zero. Tg is a kinetic parameter of amorphous material whereby using different cooling rates from the melts, different Tg values can be obtained. The fragility index is associated with the activation energy of the structural relaxation at the glass transition and their relationship is expressed in the following equation: m = Ea* / (2.303RTg) Eq. 1 where m is the fragility index of the material, Ea* is the activation energy and R is gas constant. The activation energy can be achieved by the following equation: dln(q) / d(1/Tf) = -Ea*/R Eq. 2 where q is heating rate, and Tf is the fictive temperature defined as the temperature of intersection between the equilibrium volume or entropy/temperature liquid curve and the linear extrapolation of glassy curve. It has been proved in the literature that Tf has a very similar value to Tg, therefore Tf was replaced by Tg in this study. Eq.2 is further modified as the following equation: dln(q) / d(1/Tg) = -Ea*/R Eq. 3 The activation energy can then be obtained by the regression analysis via the plot of 1/Tg against ln(q), and with value of the activation energy the fragility index (m) of the material can be calculated using Eq.1. 2. Calculation of structural relaxation time In this study, the relaxation times of model drugs were calculated using the Adam-GibbsVogel (AGV) model. The model is described as follows: τ = τ0exp (DT0/(T(1-T0/Tf))) Eq.4 where τ is the relaxation time at temperature T, τ0 is a constant (about 10-14second), D and T0 are the Vogel-Tammann-Fulcher (VTF) equation parameters which can be calculated by the following equations: D=2.303(mmin2)/(m-mmin) mmin=log(τTg/τ0)= log(100/10-14)=16 T0=Tg(1- mmin/m) Eq.5 Eq.6 Eq.7 where τTg represent the relaxation time at Tg and were assumed to be 100s as suggested in the literature (cited in the paper), and m is the fragility index as calculated above. The fictive temperature Tf has a very similar value to Tg, and therefore Tg is used in the relaxation calculation instead of Tf. 3. Calculation of interaction parameter and the prediction of drug-polymer solubility using melting point depression method The melting point depression method was derived from the Flory-Huggins lattice-based theory and was further developed into the method for the prediction of the drug-polymer miscibility and solubility. It is described by: (1/TmixM-1/TpureM) =-R/ΔHfus [lnΦdrug+ (1-1/m)Φpolymer+χΦpolymer2] Eq.8 where TmixM is the melting temperature (depressed melting point) of the crystalline drug in the mixture with the amorphous polymer, TpureM is the standard melting point of the pure crystalline drug, ΔHfus is the heat of fusion of the crystal, m is the ratio of the volume of the polymer to that of a single lattice site (defined here as the volume of the drug) and χ is the interaction parameter. Rearranging Eq.8, the linear relationship can be obtained: (1/TmixM-1/TpureM) × (ΔHfus/-R)-ln(Φdrug)-(1-1/m)Φpolymer = χΦpolymer2 Eq.9 The interaction parameter, χ, can be calculated as the slope of the linear regression between the left phase (calculated by the detected depressed melting points and the physical properties of drugs and polymers) and the right phase (Φpolymer2) of Eq.9. To further use the melting point depression method to calculate the drug-polymer solubility, it is essential to understand classic thermodynamic solubility in liquid theory. In liquid solutions, solubility occurs at equilibrium state where the chemical potential of the solute in solid state should be equivalent to that in the solution. Based on this theory, the relationship between the solubility and temperature can be written by: dlnS/dT = ΔHfus / (RT2) Eq.10 where S is the solubility of the material at temperature T in the solution, ΔHfus is the heat of fusion of the solid. With two fixed temperatures, Eq.9 can be written by: lnS = -ΔHfus /R (1/T2 – 1/T1) Eq.11 Therefore, considering T2 and T1 as TmixM and TpureM from Eq.7, respectively, the solubility of the drug in the polymer can be calculated by combining Eq.8 and Eq.11: lnSdrug = lnΦdrug+ (1-1/m)Φpolymer+χΦpolymer2 Eq.12 4. ATR-FTIR results of spin coated amorphous drugs aged under different conditions. Figure ATR-FTIR results of NH (felodipine) and NH2 (celecoxib, carbamazepine) groups from spin coated amorphous model drugs aged under (a) 0%RH at room temperature and (b) 75%RH at room temperature for different time periods 5. SEM images of the surface of 10% (w/w) HME felodipine-Eudragit® E PO and 10% HME celecoxib-Eudragit® E PO aged for 6 months under different conditions. Figure SEM images of the surface of (a) 10% (w/w) HME felodipine-Eudragit® E PO and (b) 10% HME celecoxib-Eudragit® E PO aged for 6 months under different conditions 6. DSC results of 10% (w/w) HME samples after 6 months aging under different conditions. Figure DSC results of 10% HME formulations after 6 months aging under different conditions (a) HME felodipine-Eudragit® E PO (b) HME celecoixb-Eudragit® E PO (c) HME carbamazepine-Eudragit® E PO and (d) HME fenofibrate-Eudragit® E PO