Título del Trabajo a Presentar en el XV Congreso Nacional de
advertisement

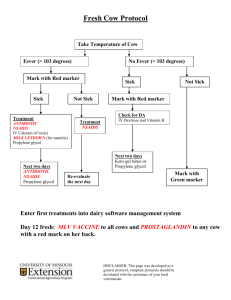
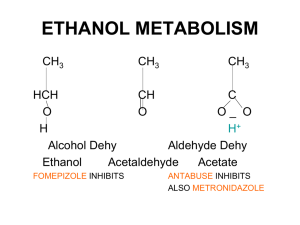
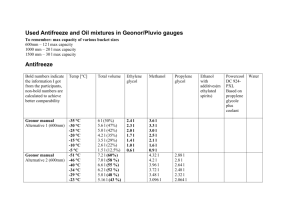
Synthesis of Propylene Glycol over Heterogeneous Catalysts S. Akyalcin (1) (1) Department of Chemical Engineering, İki Eylül Campus, 26555, Eskişehir, TURKEY +902223213550 sdogruel@anadolu.edu.tr 1. Introduction – Propylene glycol (PG) is an important intermediate for numerous chemicals, in the production of alkyd resins for paints and varnishes, in hydraulic fluids, and in heat transfer fluids. Propylene glycol has very low toxicity, therefore it is directly used in foods, pharmaceuticals and cosmetics. Propylene glycol is produced by the hydration of propylene oxide which proceeds a series-parallel reactions. During the reaction, dipropylene glycol (DPG) and tripropylene glycol (TPG) are produced as by-products [1, 2]. Propylene glycol can be produced either catalytically or non-catalytically. In the non-catalytic production of PG, the reaction is carried out at high temperature with large amount of water (12 to 20 mol water/mol propylene oxide(PO)) [1, 2]. These increase the energy consumption and the product separation cost. On the other hand, the hydration of propylene oxide can be performed at low temperature and low water/PO mol ratios in the presence of either a homogeneous or a heterogeneous catalyst. The use of a heterogeneous catalyst has inherent advantages over catalysis affected by dissolved electrolytes since they eliminate the corrosive environment and can easily be removed from the reaction mixture by decantation and filtration [3]. The synthesis of PG was carried out by the hydration of propylene oxide over different catalysts [4-6]. PG selectivity can be increased by choosing the proper catalyst. In the present work, Lewatit MonoPlus M500/HCO3- , Lewatit MonoPlus M500/OH- ,and Dowex Marathon A/HCO3- resins were tested as the heterogeneous catalysts. The performance of these ion exchange resins were compared with each other at the same reaction conditions in view of PG selectivity and catalyst activity. 3. Results and Discussion - The reaction was conducted in a pressurized batch reactor at 358 K and water/PO mol ratio of 7:1. In each case, a different type of catalyst containing the equivalent amount of HCO3- and OH- which are corresponding to 0.23 mol/L, was used. The effect of catalyst type on the conversion of PO is given in Figure 1. CONVERSION OF PROPYLENE OXIDE 1 0.8 0.6 uncatalyzed NaHCO3 Dowex Marathon/HCO3Lewatit M500/HCO3NaOH Lewatit M500/OH- 0.4 0.2 0 0 100 200 300 400 500 600 REACTION TIME, MIN Figure I. Effect of catalyst type on conversion of propeylene oxide at 358 K and water/PO mol ratio of 7:1 4. Conclusions –Lewatit MonoPlus/OH- has showed the highest catalytic activity among the others. PG selectivity in the presence of Lewatit MonoPlus/OH- and Lewatit MonoPlus/HCO3- is found to be 68 % and 95 %, respectively. 5. References [1] R.E. Kirk, D. F. Othmer, In Encyclopedia of Chemical Technology;Wiley: Toronto, Canada, 1982. [2] A.Chauvel, G.Lefebvre, Petrochemical Processes, Editions Technip, Paris, 1989. [3] M.R. Altiokka, M. R.; A. Citak, Appl. Catal., A: Gen. 141, (2003) p. 239. [4] I.A.Kozlovsky, R.A. Kozlovsky, A.V. Koustov, et al., Org. Process Res. Dev., 6, (2002) p. 660. [5] R.Z. Shaikhutdinov, L.A. Petukhov, N.V. Sapunov, et al., Kinet. Catal., 51(1), (2010) p. 50. [6] Z.Liu, W. Zhao, F. Xiao, W. Wei, Y. Sun, Catal. Commun., 11, (2010) p.675.