graduate student handbook - College of Literature, Science, and the
advertisement

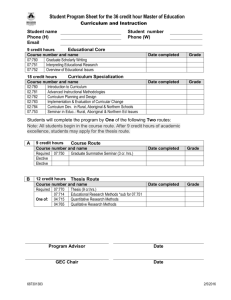
Biophysics University of Michigan GRADUATE STUDENT HANDBOOK Updated August 26, 2011 INTRODUCTION This Handbook serves as a guide to the rules and regulations of the Biophysics Graduate Program as well as that of the Rackham Graduate School here at the University of Michigan. As a student you should familiarize yourself with requirements of the Biophysics program and the requirements of the Rackham Graduate School. Throughout the Handbook references are made to Graduate School rules which can be found in their entirety in the Rackham Graduate Student Handbook. For counseling, consult with the Graduate Chair or the Student Services Administrator. Students in research groups should always consult their thesis mentor first. Biophysics at Michigan is inter-disciplinary and multi-disciplinary research, distinct from the Ph.D. Programs in Physics, Chemistry, Biological Chemistry, or Biology. It encompasses fields as different as structural biology (Xray and NMR structure determinations), biomolecular spectroscopy (NMR, IR, UV, EPR), computational biophysics (protein structure prediction, ab initio forcefield calculations), cellular biophysics (biomolecular mechanics, manipulation of single protein molecules, receptor diffusion in membranes) and biophysical chemistry (peptide design, protein folding, thermodynamics). The degree in Biophysics is conferred in recognition of independent, insightful and physically-oriented investigations of biological processes, matter or theories as demonstrated in a thesis based upon original research and creative scholarship. It is the mission of the Biophysics graduate program to help students prepare for a career in Biophysics by coordinating their course work, research endeavors, teaching commitments and financial aid. There is no independent Master of Science (M.S.) program in the Biophysics Graduate Program and the Program will not admit students intending to obtain a terminal Master of Science Degree. Related information can be found on the Biophysics website www.biop.lsa.umich.edu. This handbook supersedes the website. TABLE OF CONTENTS WHERE TO GO FOR INFORMATION ........................................................................ 3 DEPARTMENT STAFF.................................................................................................... 4 CONTINUOUS ENROLLMENT POLICY .................................................................... 5 GRADUATE SCHOOL REQUIREMENTS................................................................... 5 BIOPHYSICS GRADUATE PROGRAM REQUIREMENTS ..................................... 6 COURSES .............................................................................................................................................................................. 6 STARTING RESEARCH ....................................................................................................................................................... 6 GRADUATE STUDENT INSTRUCTORSHIP (TEACHING) ............................................................................................. 7 PRELIMINARY EXAMS ...................................................................................................................................................... 7 SEMINAR .............................................................................................................................................................................. 9 THESIS RESEARCH ........................................................................................................................................................... 10 THESIS COMMITTEE ........................................................................................................................................................ 10 GOOD STANDING ............................................................................................................................................................. 10 THESIS PREPARATION AND DEFENSE ......................................................................................................................... 11 VACATION POLICY .......................................................................................................................................................... 11 STUDENT REPRESENTATION ........................................................................................................................................ 12 MASTERS DEGREE ........................................................................................................................................................... 12 FINANCIAL INFORMATION ...................................................................................... 12 FACULTY AND ASSOCIATED FACULTY MEMBERS ......................................... 14 TYPICAL CHRONOLOGY OF PH.D. DEGREE ....................................................... 15 COURSE REQUIREMENTS ......................................................................................... 15 SAMPLE CURRICULUM .............................................................................................. 16 COURSE DESCRIPTIONS ............................................................................................ 17 A. B. C. D. E. F. G. H. I. BIOLOGICAL CHEMISTRY .................................................................................................................................... 17 BIOMEDICAL ENGINEERING ................................................................................................................................ 18 BIOPHYSICS ............................................................................................................................................................. 18 CHEMISTRY ............................................................................................................................................................. 19 MATHEMATICS ....................................................................................................................................................... 20 MOLECULAR, CELL, & DEVELOPMENTAL BIOLOGY ..................................................................................... 20 PHARMACEUTICAL CHEMISTRY ........................................................................................................................ 21 PHYSICS .................................................................................................................................................................... 21 STATISTICS .............................................................................................................................................................. 22 2 WHERE TO GO FOR INFORMATION Graduate Student Handbook This handbook is to be used as a guide to the rules and regulations that govern the graduate program both here in the Biophysics program as well as the University of Michigan. As a student you must familiarize yourself with requirements of the Department and the Rackham graduate school. Throughout the Handbook references are made to Rackham rules and regulations which can be found in their entirety in The University of Michigan Bulletin, Rackham Graduate Student Handbook on their website: http://www.rackham.umich.edu/. Email Each student will have a UM email address and account. Messages and information are sent to the graduate student group biophysics-gradstudents@umich.edu and on an as needed basis to individual students. Please check your email every day. Copy Room The copy room (#4029) is located on the 4th floor directly across from the Biophysics administrative office (#4028). If you need to make copies that are course or lab related, stop in the Administrative Office and you will be given a code for the copy machine. Once you join a lab, you will be given a lab-specific copy code to use. Graduate Mailboxes Every graduate student has their own mailbox located in the Biophysics lounge on the 4th floor (room 4041). Any mail addressed to you here in Biophysics will be put there, as well as any messages from faculty, Academic Services staff, Technical staff or Rackham will be put in your mailbox. Please check your mailbox regularly. Biophysics Student Library Our library contains various Biophysics and related texts for student use. Please see someone in the Administrative Office for a key. The usual loan period is for 1 week. If you need more time, please contact Sara in the Biophysics main office. Bulletin Board The board is located outside of the Administrative Office (4028). You will find such information as Fellowships, Scholarships, Grants offered, Job listings, and other University business pertaining to graduate students. Graduate Academic Advisor First year students will see Professor Ayyalusamy Ramamoorthy for academic counseling. After your first year, your Research Advisor/PI will be your academic counselor. 3 DEPARTMENT STAFF Administration Room Telephone* Chair Jens-Christian Meiners 4028C 4-1146 Associate Chair, Graduate Program Hashim Al-Hashimi 4022 5-3361 Administrative Manager Jane Ginopolis 4040 5-7056 Executive Secretary Ann Titus 4028b 4-1146 Financial Analyst Kim Angelopoulos 4028 4-5258 Student Services Manager Sara Grosky 4028 3-6722 Systems Administrator Tony Markel 4019 7-2146 Research Engineer Keith Shaw 3012 3-2501 * When dialing from a campus phone, only the last five digits are used 4 CONTINUOUS ENROLLMENT POLICY The Dean and the Executive Board of the Rackham Graduate School have approved the adoption of a continuous enrollment requirement for Ph.D. students at the University of Michigan, to become effective in the Fall Term 2010. Once admitted to a Ph.D. program, students will register every fall and winter term until their degree is awarded, unless they are taking an official leave of absence. Please consult your Rackham handbook or Rackham’s website for more information: http://www.rackham.umich.edu/policies/continuous_enrollment/. RACKHAM GRADUATE SCHOOL REQUIREMENTS The basic requirements for the degree of Doctor of Philosophy set by the Rackham Graduate School include: 1. 2. 3. 4. 5. 6. Adherence to the Continuous Enrollment Policy Completion of at least 18 hours graded graduate coursework on the Ann Arbor campus Satisfactory academic standing (“Good Standing”) Attainment of Candidacy (passing of Preliminary Exams) Appointment of a Thesis Committee to supervise the student’s program and progress in research Approval of the written dissertation by the Thesis Committee and Rackham, and a final examination by the Thesis Committee 7. Minimum of four cognate credit hours – courses taken outside the Home Program. Undergraduate transcripts/credentials need to have been submitted to Rackham within seven years from the start of the current program. BIOPHYSICS CREDIT REQUIREMENTS The minimum requirement for the Ph.D. degree is seven (eight including the Final Term – six with a relevant Masters) full time terms of study and research beyond the bachelor’s degree. A student is considered full time with registration of 9 hours per term (8 hours after Candidacy). A graduate student research or teaching assistant must be a full-time student. GSRA’s or GSI’s can be full time with 6 hours. A minimum of 18 credits must be accumulated prior to admission to Candidacy. These hours include all regular required program courses, any courses in which the student registers and pays fees as a visitor but exclude Biophysics 890 (Rotation Research) and 990 (Pre-Candidate Research). A minimum of 6 credits each for 890 and 990 must also be completed in order to achieve candidacy. The number of hours registered for Biophysics 990 may range from one to eight per term as approved by the thesis mentor. A student must register for a total of 9 hours per term prior to Candidacy. Following admission to Candidacy, the student must complete a minimum of 48 credit hours of 995. This balance between the pre-Candidate and Ph.D. candidate-hours is made up by eight hours per term of registration in Biophysics 995. A student must register for a total of 8 hours as a candidate. Students are allowed one extra course on top of the 995 research credits, per Rackham guidelines. Students should register for Fall & Winter terms only. 5 BIOPHYSICS GRADUATE PROGRAM REQUIREMENTS Please note that Biophysics requirements include and supersede the Rackham requirements. COURSES It is the goal of the Program to make sure the student acquires a solid background in biology, biochemistry, chemistry, physics, and biophysics, by including a selection of courses on the graduate or senior undergraduate level. Establishing a solid academic foundation is especially important in a rapidly changing interdisciplinary field such as Biophysics. In total, six courses are required. All students are expected to complete the four core biophysics courses (BIOPHYS 520, 521, 550 and 595). Because of the interdisciplinary nature of Biophysics, students enter the program from a variety of undergraduate backgrounds. Most students, unless they have an unusually strong background in biology, will be expected to complete one course in cell biology and one course in biochemistry. Students that lack a strong foundation in the physical sciences should take appropriate advanced undergraduate courses or introductory graduate courses in quantum mechanics or quantum chemistry and in thermodynamics or statistical physics. Pre-candidates must be registered for a minimum of 9 credit hours. The course requirements begin on page 14, and their descriptions start on page 16. Each course is 2-4 credits except as noted. Courses are counted towards the Biophysics Requirements only when they are passed; for undergraduate courses (400-level) passing grade is “C” or above, in graduate courses (500-level and above), passing grade is “B” or above. However, be aware that failed courses keep counting towards your cumulative grade-point-average (GPA), which has to remain “B” or above at all times. (See “Good Standing” below.) Students who are Doctoral Candidates (i.e. who have passed their preliminary exams) can take one additional course for credit. Follow the rules given in the Rackham Handbook. Other classes may be audited (contact the instructors). Visit is the Graduate version of audit (VI). Taking extra courses before and after Candidacy must be discussed with the Thesis Mentor or with the Graduate Chair when the student has not chosen a home lab. Grades in research courses accepted by the Graduate School are “S” (satisfactory) and “U” (unsatisfactory). No credit is given for a “U” but RFT’s are earned if applicable. An “I” grade may be given in any lecture or laboratory course when a minor part of the course work remains undone at the end of the term. If the work is made up within two complete semesters, a supplementary report of the appropriate letter grade may be filed; after the second semester the supplementary report will not be accepted and the “I” remains permanently on your record. Check in the Rackham Handbook for specific information on the Incomplete Policy. An “I” always remains on the record. STARTING RESEARCH The Ph.D. signifies the completion of a significant body of original publishable research, performed under the supervision of a research advisor. The choice of a research advisor and thesis project is a major decision. This choice is facilitated by a rotation program, which allows students to explore various research laboratories and areas of Biophysics research during their first year. You should register for at least one term of Biophysics 890, Introduction to Research, consisting of a laboratory rotation in the laboratory of any Program faculty member upon mutual agreement. It is recommended that you carry out at least two semesters of Biophysics 890 rotations. Even students sure of their choice of thesis advisor are encouraged to take advantage of this opportunity to broaden their exposure to different research efforts. Students are encouraged to choose their thesis lab before the start of their second year. You may join in the laboratory of any of the regular or associated faculty. Since the thesis mentor will be responsible for the majority of stipend, tuition and fringe benefits expenditures, it behooves the student to consider the financial position as well as the scientific interest when choosing a lab. You should register for Biophysics 990 (Pre-Candidate Research) during each semester of dissertation work as long as you are a pre-Candidate. You must present an oral prospectus of your research project to your thesis committee after achieving candidacy. This is not an exam, but a review to ensure that the project is indeed in the field of Biophysics, broadly defined, and of an appropriate scope. After that, you should discuss your progress annually with your thesis committee. 6 You may decide at some point that you want to switch thesis labs; this is permissible. The decision is consequential because the choice of a lab amounts to the choice of a research field that will affect much of your future career. You are encouraged to switch only after serious reconsideration. The student and the thesis mentor are jointly responsible for following the Program and Graduate School requirements for the Ph.D. The mentor’s responsibilities begin at the time of his/her agreement to accept the student for research. In addition to supervising the research, the thesis mentor is expected to advise the student on course elections, examinations, independent study pertinent to his/her general development as a scientist and any other matters affecting his/her general progress toward a degree. GRADUATE STUDENT INSTRUCTORSHIP (TEACHING) Although there are no formal teaching requirements, as part of their training students are strongly encouraged to teach at least one semester as a Graduate Student Instructor (GSI) in Biophysics, Chemistry, Biology, Biochemistry, or Physics. This experience is especially important for those interested in a future career in academia, although all students can gain from the opportunity for presenting technical material in a pedagogical context. It may happen that you are asked to teach in later stages of your study as well, depending on financial resources of your thesis mentor (see “Financial Support”). Students are strongly encouraged to serve as teaching assistants in a form that includes direct contact hours with a class (rather than grading). It is mandatory for students assigned teaching positions for the first time to take a one-credit GSI Training Course associated with the program in which you are teaching. This course is usually offered the last week in August. PRELIMINARY EXAMS Students are required to pass a three-part preliminary exam in order to attain candidacy. The three parts of the exam cover Biochemistry and Cell Biology, Physics and Physical Chemistry, and Biophysics. The Biochemistry/Cell Biology part will be at the level of Stryer’s Biochemistry. The Physics/Physical Chemistry part will cover Quantum Mechanics/Quantum Chemistry and Thermodynamics/Statistical Mechanics at the advanced undergraduate level, combined with Classical Mechanics and Electricity and Magnetism at a General Physics (with calculus) level. The Biophysics part will be an original research proposal, presented to a panel of three faculty members, addressing a question of current relevance in biophysics. These three parts can be taken and passed independently of each other. The Biochemistry/Cell Biology and the Physics/Physical Chemistry parts will be given in the winter term. It is recommended that students with the appropriate undergraduate background take these parts during their first semester, so as to establish competency in these particular areas. The Biophysics (oral) part will be given in May. It is expected that students will pass this part at the end of their second year. Students can take the exam parts as often as they are given. Students are expected to pass the preliminary exam and required coursework and achieve candidacy by the start of their third year. A preliminary list of topics to be covered by the exams is included below: Biochemistry and Cell Biology Preliminary Exam Topics At the level of Stryer’s Biochemistry Proteins o Amino acids o Peptide bond o Levels of protein structure o Sequencing of proteins o Characterization of proteins o Globular proteins – hemoglobin o Fibrous proteins – Collagen, silk, wool o Protein folding o Enzymes o Enzyme kinetics 7 Michaelis-Menten Types of inhibition Mechanisms Regulation Lipids o Triglycerides o Phospholipids o Steroids o Membranes Carbohydrates o Glycolysis o Gluconeogenesis o Glycogen o Citric Acid cycle o Pentose phosphate pathway o Oxidative phosphorylation Nucleic Acids o Bases o DNA structure and replication o RNA structures, transcription, splicing o Protein synthesis Cell Biology o Cell architecture Nuclear structure Membrane structure o Cell trafficking o Signal transduction o Transport o Cell replication o Cell motility Physics and Physical Chemistry Preliminary Exam Topics Classical Mechanics (at the level of General Physics (with calculus): Halliday and Resnick) o Forces, torques, momentum and energy of linear and rotational motion o Levels of protein structure o Gravitational attraction and potentials o Oscillatory motion including damped and forced harmonic oscillators o Coupled harmonic oscillators o Central force motion o Kinematics of two-particle collisions o Non-inertial reference frames; Coriolis force Electricity and Magnetism (at the level of General Physics (with calculus): Halliday and Resnick) o Electrostatics with dielectrics and multipoles o Maxwell’s equations in integral form o Electromagnetic waves o Polarization o Reflection and refraction o AC and DC circuits Quantum Mechanics (at the level of upper-class undergraduate physics or physical chemistry) o Uncertainty principle o DeBroglie wavelength o Schroedinger equation, expectation values, probability densities o Bound states in a square well, atom, and harmonic oscillator o Tunneling o First-order time-independent and time-dependent perturbation theory o Valence Bond theory and molecular orbitals o Delocalized orbitals Statistical Mechanics and Thermodynamics (at the level of upper-class undergraduate physics or physical chemistry) o First and second laws of thermodynamics and simple applications o Free energies, definitions and uses o Statistical definition of entropy o Ensembles o Partition functions o Kinetic theory of gases in equilibrium 8 o o Phase transitions Boltzmann distribution Biophysics Preliminary Exam Guidelines The goal of the Biophysics Prelim Exam is to evaluate a student’s preparation for and ability to successfully complete a Ph.D. thesis. Successful completion of a Ph.D. requires the ability to synthesize information from a variety of sources. The Prelim exam is intended to evaluate a student’s ability to bring together the knowledge learned in formal coursework and from readings of the research literature and to combine this information to produce an original research proposal. This proposal (no longer than 10 pages single spaced, excluding figures and references) will be evaluated by a candidacy committee. The committee will then meet with the student in an oral exam at which the student will defend the proposal. Selection of topic. Students are encouraged to make the proposal relevant to their interests, ideally a project that could broaden and extend the work of their lab. The candidate should submit a one-paragraph summary of their proposed Original Research Proposal to the Graduate Chair no later than the middle of their 4th semester in residence (see the timeline below). Once this is approved, the candidate may begin preparation of the full proposal. Scope of proposal. The Original Research Proposal should utilize a variety of biophysical methods to address a question of current scientific interest. Methodology should be described at the level of that discussed in Biophysics 520 and 521. The proposal should include a review of the relevant background literature, although this need not be an exhaustive review. The maximum length for the proposal is 10 pages, single-spaced, exclusive of figures and references. References should be in the NIH style (include titles) and may not exceed 3 pages. Candidates should note that longer proposals are seldom better proposals; the maximum length should not be taken to represent the ideal length. Preliminary (Candidacy) exam committee. A committee of at least 3 Biophysics faculty members and one cognate (non-Biophysics) faculty member will review the proposal. This committee should not include the candidate’s thesis advisor. With the addition of the thesis advisor, the prelim exam committee will often be identical to the candidate’s Ph.D. committee (although this is not required). The Preliminary Exam Committee must be approved by the Graduate Chair. Timeline. The timeline assumes that students start in the fall and that they complete candidacy requirements during their 4th semester (the winter semester of their second year). This timing takes advantage of the 1-month grace period offered by Rackham for the Prelim (prelim month of May). Students that have a different schedule should consult the graduate chair regarding timing. 1. Selection of topic for proposal – by the end of March 2. Completion of proposal – by the last day of finals for the winter semester. 3. Defense of proposal to faculty committee – before the end of May after their 4th semester. 4. The 1 month grace period (May) for Prelims is for students who are registered full time the previous Fall and Winter terms. SEMINAR Students are required to present two seminars to faculty and fellow students. This offers you practice in presenting a scientific lecture. One seminar is given as a seminar in the regular Biophysics Seminar Series and takes place somewhere in the middle of your graduate career. It is recommended that you present your own research. Should you decide to select a topic that is not your own research, consult with the Graduate Chair for approval and/or assistance. The other seminar is a requirement for your thesis defense. You may be invited to give yet another seminar in the regular Biophysics Seminar Series or other departments in the later stages of your career. Students are encouraged to accept these invitations as they help develop good communication skills. 9 In addition to presenting a seminar, all students are required to attend the Biophysics Seminar Series. The serious student will take advantage of all learning opportunities, and the Seminar Series represents excellent sources of up-to-date results and ideas. THESIS RESEARCH When the formal course requirements and preliminary exams are completed successfully, then you will have reached Candidacy status. Once you have obtained Candidacy, your main activity in Biophysics will be thesis research. Every semester you should register for Biophysics 995 for 8 credit hours. You will also want to attend many of the numerous specialized lectures and seminars at Michigan, and you may also want to take or audit additional courses of interest to you. Normally, Candidacy should be achieved before the beginning of the third year. The thesis research should involve original and significant advances of our understanding of an important area in Biophysics. It is expected that your work will result in papers published in peer-reviewed scientific journals. In fact, the experiencing of presenting your work in written and oral form is an important part of the graduate experience. THESIS COMMITTEE As the outlines of the research and preliminary results become clear, it is necessary to form a Thesis Committee and present an introduction and progress report to them. This should be done as soon as possible after achieving candidacy. And the list of final Dissertation Committee Members must be submitted to Rackham for APPROVAL at least 6 months prior to the oral defense. The members of the Thesis Committee can form a valuable scientific resource, and provide an opportunity for you to take advantage of the broad areas of expertise present at the University of Michigan. In some cases, your research can be a collaboration among you, your main research advisor, and another committee member. The composition of the thesis committee is subject to the approval of the Biophysics Program Committee or Program Chair, as well as Rackham. Additional meetings with the Thesis Committee may be requested by you, your research advisor, the Thesis Committee, or the Biophysics Program Committee. At the very least, you should meet once a year; it is recommended that you meet twice a year when approaching thesis defense. The Biophysics Program has rules for the constitution of the Thesis Committee. These rules are consistent with Rackham requirements, but they go beyond the general Rackham rules: 1) The committee should consist of at least five members. 2) Your thesis advisor/mentor is generally the Thesis Committee Chair. In some cases, there may be two Co-Chairs. 3) A research scientist with an earned doctorate from an accredited institution can serve on the Thesis Committee. They can be the sole Thesis Committee Chair only if they have been so approved by Rackham and the Chair of the Biophysics program. 4) At least three of the five committee members must be regular Academic Rackham Faculty members; i.e., not Research Scientists. Two of these three should be chosen from the list of regular Biophysics program faculty members. One of these three must be other than a regular Biophysics faculty member. These requirements also satisfy the requirements for those Biophysics graduate students who are in the Molecular Biophysics Training Program; in that case, just be sure that at least three faculty members are also in the Molecular Biophysics Program. GOOD STANDING It is critical to maintain a record of Good Standing in the Biophysics Graduate Program. Only students who are in good standing are considered for financial support. The Rackham Graduate School’s definition of “Good Standing” is detailed in the Rackham Handbook. 1 0 “Good Standing” in the Biophysics Graduate Program includes and supersedes the Rackham requirements and is defined as a cumulative grade point average of “B” or better for all courses applied toward the degree program, passing grades in all courses applied toward the degree program, whether these courses can be defined as “cognates” or not, having entered into research with a Thesis Advisor affiliated with the Biophysics Graduate Program by spring term of the second year, maintaining satisfactory progress in dissertation research (Biophysics 990 and/or 995) with minimum grades of “S” as reported by the student’s Thesis Advisor, and A student whose cumulative grade point average falls below a “B” in a given term or half-term will be placed on probation for the following term or half term, or may be denied permission to register. See the Rackham Handbook. THESIS PREPARATION AND DEFENSE Detailed instructions about the preparation and defense of the Dissertation are in the Dissertation Handbook, available online at Rackham’s website. In general, the Dissertation is a comprehensive treatment of the Thesis Research performed by the student. It is possible for the Dissertation to include material from journal articles previously published by the student, however the Dissertation should also include contextual information regarding the significance of the question being addressed, a discussion of other approaches used by previous researchers, and the importance of the Thesis research. Following the submission of the Dissertation to the Thesis Committee, the student must defend the Dissertation in an oral presentation. The student must have a pre-defense meeting with the Office of Academic Records and Dissertations at least 10 working days before the defense. The student can register online for this, and must be registered to Defend and finish all requirements. The oral defense generally consists of an open presentation of the thesis research to the University Community followed by a closed session with the Thesis Committee. At least four members of the Thesis Committee must be present at the oral defense. Including the Chair or one Co-Chair and Cognate Member. All members are required to read and comment on the submitted Dissertation before the Defense. VACATION POLICY & HOLIDAYS Graduate students should discuss proposed vacation plans with their thesis advisor. For first year students, this means your current rotation mentor and the Associate Chair for the Biophysics Graduate Program. Time between semesters or academic quarters is considered an active part of the training period. The University observes the following holidays: New Year’s Day Memorial Day Independence Day Labor Day Thanksgiving Day The day following Thanksgiving Christmas University-designated holidays will be observed on the calendar day on which each falls except that holidays falling on Sunday will be observed on the following Monday and holidays falling on Saturday will be observed on the preceding Friday. 1 1 STUDENT REPRESENTATION The Biophysics program has annual elections in which they elect candidates to represent the graduate students for the following: One student serves on the Admissions Committee (One year term) One student serves on the Curriculum Committee (One year term) One student serves as the Graduate Student Representative at the Biophysics faculty meetings (One year term) MASTERS DEGREE There is no independent Master of Science (M.S.) program in the Biophysics Graduate Program and the Program will not admit students intending to obtain a terminal Master of Science Degree. However, the degree can be granted in the extenuating cases of students who have unsuccessfully attempted to pass the preliminary examination requirements. It can also be conferred as a non-terminal degree to students who are working to complete the Ph.D. degree. The Master of Science Degree in Biophysics, when granted by Biophysics Graduate Program, requires successful completion of specific course requirements (4 courses in Physics and Chemistry, 2 courses in Biological Chemistry, 1 course in Cell Biology, and 2 courses in Biophysics) with a minimum of 24 credit hours of course work, average “B”, and 4 credit hours of cognate studies, passing grade “C-”. 990, 995 or “VI” courses can not count towards this total – student should also see the Rackham Handbook section on Masters Degrees. FINANCIAL INFORMATION 1. POLICY ON FINANCIAL SUPPORT The Biophysics Graduate Program is committed to seek continued support for your stipend, tuition, and health insurance throughout your graduate training. To be eligible for such financial support, students must be in “Good Standing” (see above). Students are expected to continue to make progress in their thesis research independent of the source of their funding. Financial support will be provided by Biophysics for full coverage of tuition and health care in your first year (12 months); in addition, a stipend in the amount of $25,553 is provided. Following the choice of a thesis mentor, support will be provided by some mix of the following: (1) a Research Assistantship support by an individual grant to the faculty member; (2) a Teaching Assistantship (GSI) in a relevant academic department; or (3) an extramural fellowship. Fellowship support may be in the form of a fellowship awarded directly to the student by a national agency (e.g. NIH or NSF), or a research foundation or University Fellowship (e.g. Rackham Research Partnership, Rackham Merit Fellowship, or Howard Hughes Fellowships) awarded on a competitive basis. In addition, the Molecular Biophysics Training Grant Program, funded by the NIH, provides fellowship opportunities for U.S. citizens or permanent residents. Participation in the Training Grant may require minor modifications in the curriculum. These possibilities should be discussed with your Thesis Mentor and the Program office. Fellowships usually carry a full tuition waiver. Graduate students holding at least a quarter-time appointment as a Graduate Student Instructor (GSI) or Research Assistant (GSRA) will have the full tuition waived. However, you will be liable for mandatory or registration fees, currently approximately $95 per term. Students in the Molecular Biophysics Training Grant Program will have these fees waived. Stipend Payment Schedules. Stipends for teaching, research and fellowship appointments are paid in equal installments depending on whether the appointment is for a term or the academic year (e.g., four per term or twelve per year). Students who have GSRA or GSI appointments are paid on the final day of the month. Students who are on fellowship can expect their checks to be deposited somewhere in the middle of the month according to 1 2 the payment schedule of the Office of Financial Aid. 2. LOANS Loan funds administered through the Office of Financial Aid (2011 SAB) are available to meet the needs of any educational expense for students while enrolled in the University. The need for this financial aid must be clearly established by providing a complete statement of the applicant’s financial resources and expenses for the academic year. Loans are NOT available for any non-education expense which is normally financed by a commercial lending institution, nor are they available for the repayment of previously incurred indebtedness. Short term loans up to $250 are considered by the Office of Financial Aid. Departmental short term loans for emergencies are available. See the Student Services Administrator for details. 3. INCOME TAX LIABILITY Graduate Student Instructor stipends are currently considered remuneration for services performed and, as such, are subject to withholding and income tax. Under the income tax law of 1986, stipends for Research Assistants, fellowships and other forms of student aid are now subject to income tax. Current practice is subject to review by the IRS and may change at any time. Consult with the district office where you file your income tax return for interpretations of rules. 1 3 FACULTY AND INTERDISCIPLINARY PROGRAM FACULTY MEMBERS Name Al-Hashimi, Hashim Ballou, David Banaszak Holl, Mark Bardwell, James Biteen, Julie Brooks, Charles Carlson, Heather A. Chen, Zhan Cierpicki, Tomasz Fierke, Carol Gafni, Ari Hunt, Alan Joglekar, Ajit Kerpolla, Tom Kopelman, Raoul Kubarych, Kevin Lehnert, Nicolai Lei, Ming Lubensky, David Marsh, E. Neil Meiners, Jens-Christian Meyhöfer, Edger Ninfa, Alexander Ogilvie, Jennifer Palfey, Bruce Pecoraro, Vincent Penner-Hahn, James Raghavan, Malini Ramamoorthy, Ayyalusamy Saper, Mark Savit, Robert Sension, Roseanne Sivaramakrishnan, Sivaraj Skiniotis, Giorgios Smith, Janet Steel, Duncan Sunahara, Roger Swanson, Joel Tesmer, John Trievel, Ray Veatch, Sarah Verhey, Kristen Walter, Nils Wang, Shaomeng Xu, Zhaohui Young, Matthew Zand, Robert Zochowski, Michal R. Department/s Biophysics, Chemistry Biological Chemistry Chemistry, Macromolecular Science & Engineering Biology Chemistry Chemistry, Biophysics Medicinal Chemistry Chemistry Pathology (Medical School) Chemistry, Biological Chemistry Biophysics, Biological Chemistry Biomedical Engineering Cellular & Developmental Biology Biological Chemistry Chemistry, Physics Chemistry Chemistry Biological Chemistry Physics Chemistry Biophysics, Physics Mechanical Engineering Biological Chemistry Biophysics, Physics Biological Chemistry Chemistry Biophysics, Chemistry Microbiology & Immunology Biophysics, Chemistry Biophysics, Biological Chemistry Physics Chemistry Cellular & Developmental Biology Biological Chemistry Biological Chemistry Biophysics, Physics, Engineering Pharmacology Microbiology & Immunology Pharmacology Biological Chemistry Biophysics Cellular & Developmental Biology, Life Sciences Institute Chemistry College of Pharmacy Biological Chemistry Biological Chemistry, Bioinformatics Biological Chemistry, Macromolecular Science & Engineering Biophysics, Physics 1 4 TYPICAL CHRONOLOGY OF PH.D. DEGREE Year 1 Complete at least 4 courses. Two research rotations (Biophysics 890 – Fall and Winter terms). Initial preliminary exam(s). Select thesis mentor. Start thesis research. Year 2 Complete remaining courses. Continue thesis research (Biophysics 990). Complete preliminary exams. Achieve Candidacy status. Year 3 Continue thesis research (Biophysics 995). Select Thesis Committee (by late August). Present research prospectus. Present individual seminar. Year 4-5 Continue thesis research (Biophysics 995). Annual report to committee. Dissertation defense. COURSE REQUIREMENTS All students must take a total of 6 courses, including the four core courses: Biophys 520 (Fall), Biophys 550 (Fall), Biophys 521 (Winter) and Biophysics 595 (Fall). The other 4 courses will be decided on in consultation with your advisor (see below). In the first year, you will also need to register for research rotations: Biophys 890 (Fall and Winter). In the second year, when you’ve chosen an advisor, you’ll register for Biophys 990. These research courses – Biophys 890 and 990 – don’t count toward the 6 required courses. Students with a background in Physics will probably need to take: Biochemistry: Biolchem 550 (Fall) Additional courses could be: Cell Biology: MCDB 428 (Winter, Biological focus) or BiomedE 418 (Winter, Quantitative/Engineering focus) Macromolecular Structure/Function: Chembio 501 (Fall) / Chembio 502 (Winter) or BiolChem 515 (Fall) Dynamical Processes in Biophysics: Physics 417 (Winter) 1 5 Students with a background in Biology will need to obtain a solid background in Mechanics, Electricity and Magnetism, Statistical/Thermal Physics, and Quantum Mechanics. Choose courses in your weakest area(s): Mechanics: Physics 401 (Fall or Winter – Intermediate Mechanics) E&M: Physics 405 (Fall or Winter – Intermediate Electricity and Magnetism) Statistical/Thermal: Chem 463/575 (Fall – Thermodynamics), or Chem 576 (Winter – Statistical Mechanics), or Physics 406 (Fall or Winter – Statistical and Thermal Physics) Quantum: Chem 461/570 (Fall or Winter – Physical Chemistry) or Physics 453 (Fall or Winter – Quantum Mechanics) Once you’ve passed the prelims/chosen a lab, there are a number of courses related to specific areas of research, which should probably be chosen after discussion with your advisor/thesis committee, for example: X-ray Crystallography: Biophys 602 NMR: Biophys 503 Kinetics: Chem 673 Spectroscopy: Chem 580 And other courses in Math (463), Statistics (401), Bioinformatics, Applied Physics, etc. Some suggested electives are listed below under “Course Descriptions.” SAMPLE CURRICULUM Physics Background 1st Fall: Biophys 890 Biolchem 515 Biophys 520 (may be a challenge – find people to study with) or any necessary Physics Biophysics 550 1st Winter: Biophys 890 Biophys 521 Cell Biology 2nd Fall: Biophys 990 Biophys 520 if haven’t yet taken it and/or Macromolecular Structure/Function or Research-related course or both Biophys 595 if haven’t yet taken it 2nd Winter: Biophys 990 Research-related course if haven’t yet taken 6 courses Biochemistry Background 1st Fall: Biophys 890 Biophys 520 Biophys 550 Quantum or Statistical/Thermal or other Physics 1st Winter: Biophys 890 1 6 Biophys 521 Quantum or Statistical/Thermal or other Physics 2nd Fall: Biophys 990 Biophys 595 if haven’t yet taken it More Physics if necessary or Research-related course or both 2nd Winter: Biophys 990 Research-related course if haven’t yet taken 6 courses COURSE DESCRIPTIONS For more detailed and current information about courses please call the Student Services Administrator or check the website of each department, or check the various course guides (i.e. LSA, Engineering) available online. In many cases, a current short description of courses is maintained by the BGP Student Services Administrator. LSA Course Guide: http://www.lsa.umich.edu/cg/ Engineering Course Guide: http://www.engin.umich.edu/students/current/academics/courses/ College of Pharmacy Course Guide: http://www.umich.edu/~pharmacy/students/courses.html Biomedical Sciences Course Descriptions (in PIBS Curriculum Guide): http://www.med.umich.edu/pibs/documents/pdf/2005CurriculumGuide.pdf Department Affiliate Student Services Email Administrator Biological Chemistry ................................ Beth Goodwin ........................................... egoodwin@umich.edu Biomedical Eng……… Maria E. Steele... msteele@umich.edu Chemistry .................................................. Margarita Bekiares pappas@umich.edu Chemical Biology……. Laura Howe lkhowe@umich.edu Mathematics…… Tara McQueen tarac@umich.edu Micro. & Immunology .............................. Heidi Thompson ....................................... heiditho@umich.edu Mol. Cell & Dev. Biol. .............................. Mary Carr ................................................. carrmm@umich.edu Pharmacy .................................................. Maria Herbel ............................................. mariamh@umich.edu Physics ...................................................... Christina Zigulis czigulis@umich.edu PIBS .......................................................... Michelle Melis msmtegan@umich.edu Phone Website 764-8594 647-1091 764-7278 763-7175 764-7436 647-6742 615-1635 615-6326 764-5539 615-6538 http://www.biochem.med.umich.edu/biochem/ http://www.bme.umich.edu/ http://www.umich.edu/~michchem/ http://www.chembio.umich.edu/ http://www.math.lsa.umich.edu/ http://www.med.umich.edu/microbio/ http://www.mcdb.lsa.umich.edu/index.php http://www.umich.edu/~pharmacy/index.html http://www.lsa.umich.edu/physics/ http://www.med.umich.edu/pibs/index.html The following courses illustrate the range of approved offerings at the time of the assembly of this handbook. Some courses may have more detailed information than others. Courses change every year, be sure to contact the Biophysics Student Services Office about their existence. A. BIOLOGICAL CHEMISTRY 515. Introductory Biochemistry. (3-4 hrs, Fall). Prereq: 2 semesters of organic chemistry. A one-term introductory biochemistry course that covers the biochemistry of the living state, the chemistry of amino acids, proteins, nucleic acids, carbohydrates, lipids, and steroids, energy transformations and chemical reactions in living cells, function of immune system and action of hormones, and self-regulation and self-replication of living organisms. Students may elect to participate in BIOLCHEM 491, a weekly seminar presented jointly by clinicians and basic scientists, which correlates biochemistry lecture material with human disease through the use of clinical case studies. 550. Protein Structure & Function. (3 hrs, Fall). This course will relate protein structure to various aspects of protein function. The course will begin with a general introduction to threedimensional protein structure including discussion of structure determination methods and forces in protein structure and stability. Significant sections of the course include (i) binding and allosterism, (ii) enzyme catalysis, (iii) protein-nucleic acid interaction, and (iv) signal transduction and membrane proteins. The emphasis will be to relate details of structure to the function of the proteins discussed. The course will include a molecular graphics component aimed at hands-on experience for the students. 1 7 576. B. C. Signal Transduction. (1 hr, Winter). Prereq: Biochem. 550 or 570; or permission of instructor. A review of hormone and neurotransmitter receptors as well as the cellular effectors that are regulated by receptor activation. Oncogene products as signal transducers and the interaction of the known signaling pathways are also covered. The various techniques used to study signal transduction as well as important experimental strategies employing these techniques will also be presented. Lecture. BIOMEDICAL ENGINEERING 417. [EECS 417.] Electrical Biophysics. (4 hrs, Fall). Prereq: EECS 211 or 314; [concurrently] EECS316. Electrical biophysics of nerve and muscle; electrical conduction in excitable tissue; quantitative models for nerve and muscle including the Hodgkin Huxley equations; biopotential mapping, cardiac electrophysiology, and functional electrical stimulation; group projects. Lecture and recitation. 418. Quantitative Cell Biology (4 hrs, Winter) Prerequisite: Biology 310, 311, Biochemistry 415, 451, 515, Physics 240, Math 216, Chemistry 130. This course introduces the fundamentals of cell structure and functioning. The goal is to provide a general background in cell biology, with emphasis placed on physical aspects that are of particular interest to engineers. 525. Cellular and Molecular Networks (3 hrs, Winter) Prerequisite: Biol 105 or Biol 112 and Math 215. This course is designed to equip the student with appropriate concepts and techniques for the quantitative analysis of the integrated behavior of complex biochemical systems. A general approach is developed from the basic postulates of enzyme catalysis and is illustrated with numerous specific examples, primarily from the microbial cell. BIOPHYSICS 503. Macromolecular NMR spectroscopy. (3 hrs). Prereq: Biophys 520/521, Bio.Chem 550 or 570, Chem 570. Quantum mechanical treatment of 1-D, 2-D and 3-D NMR, applied to biological macromolecules, mainly proteins. NMR pulse sequence design, data interpretation and structure calculations. Relaxation theory. Both solution and solid NMR will be treated. 520. Biophysical Chemistry I. (3 hrs, Fall). Prereq: Chem 575, and Bio.Chem 415 or Chem 451 or Chem 215. In general, permission of the course director. This course offers an overview of protein and nucleic acid structures. Intra- and inter-molecular forces, helix-coil transitions and protein folding will be treated in a thermodynamical context. Thermodynamics of solutions, configurational statistics, ligand interaction, multi-site interactions and cooperativity are treated in depth. Calorimetry and kinetics of protein ligand binding is discussed. Experimental and computational molecular dynamics. 521. Biophysical Chemistry II. (3 hrs, Winter). Prereq: Chem 570, and Bio.Chem 415 or Chem 215. In general, permission of the course director. This course gives the background and applications of several physical techniques used in biophysical research. General principles of spectroscopy will be explained. Macromolecular structure determination by X-ray diffraction and two-dimensional NMR will be treated in fair detail. IR, Raman, CD, EXAFS, EPR will be introduced. Electron, light and scanning microscopies and optical tweezers will be introduced. In addition, each instructor will be encouraged to give a demonstration of his technique in an informal setting. 550. Intro to Biophysics Lab. (3 hrs, Fall) This course teaches essential laboratory skills in Biophysics. Experiments cover sample preparation techniques, modern research methods and computational techniques. The final project will allow students to explore a topic of interest in greater depth. 595. Professional Development in Biophysics. (3 hrs, Fall) This course teaches professional skills such as writing research articles, reviews, grant proposals, and preparing and giving poster presentations and scientific talks. The scientific publishing process, including peer review, will be discussed and ethical rules and considerations explored. All students will draft an application for an NSF Graduate Fellowship. 602. Protein Crystallography: Principles of Macromolecular Crystallography (3 hrs, Winter) Prereq: Physical Chemistry Fundamental of the methods for determining 3-dimensional structures of large molecules by X-ray crystallography. Aimed at students who expect to use crystallography as a major tool for their research, and at those who want in-depth knowledge of the methods in order to analyze structure data. 608. Biophysical Principles of Microscopy. (3 hrs, on demand). Prereq: Physics 405; or permission of instructor. This course covers the fundamental physics and math behind modern microscopies, with applications in the biological/biochemical sciences. Included are phase, differential interference, fluorescence, confocal, 3-D imaging, scanning, and electron microscopies. 801/802. Molecular Biophysics Seminar Series. Molecular Biophysics Training Grant students and non-trainees meet informally to discuss their research. As this seminar is taken for credit, students should register for it at the beginning of the semester. Non-trainee Biophysics students should register for 802. 1 8 D. CHEMISTRY 451. [Bio.Chem 451.] Introduction to Biochemistry I. (4 hrs, Fall) Prereq: Chem 215, Biol 152 or 195, and Math 115. This course is the first in a two-term sequence designed for students who are concentrators in biochemistry. Emphasis is on developing the capacity of the students to think about complex biological processes in terms of the underlying chemistry. An introductory section on proteins is followed by sections on enzymes and coenzymes. The discussion of biochemical energetics includes sections on glycolysis, the tricarboxylic acid cycle, electron transport, photosynthesis, and carbohydrate metabolism. The course has three lectures and one discussion per week. There are three hour exams and a final exam. 452. [Bio.Chem 452.] Introduction to Biochemistry II. (4 hrs, Winter). Prereq: Chem 451. This course is the second in a two-term sequence designed for students who are concentrators in biochemistry. Emphasis is on developing the capacity of the students to think about complex biological processes in terms of the underlying chemistry. Initially nucleic acids and nucleotides are discussed. The biosynthesis of amino acids and their utilization in cellular metabolism including protein synthesis serves as a primer for an introduction to biochemical genetics and virology. 501. Chemical Biology I (3 hrs, Fall) This course will provide a high-level overview on the structure, function and chemistry of biological macromolecules including proteins, nucleic acids and carbohydrates. Topics include protein and nucleic acid folding, energetics of macromolecular interactions (kinetics and thermodynamics), and mechanistic enzymology. Using specific examples from the current literature, each topic will stress how chemists have used molecular level tools and probes to help understand the specific systems under study. The over arching theme in this course will be that structure and function are intimately linked. 502. Chemical Biology II (3 hrs, Winter) This course is a continuation of CHEMBIO 501. The basic concepts obtained in CHEMBIO 501 will be applied to and demonstrated in three broad areas of interest to both chemists and biologists. The first topic will discuss combinatorial methods including SELEX and gene shuffling, combinatorial organic synthesis, high throughput screening and chemical genetics. The second topic will focus on signal transduction, emphasizing general concepts (at the molecular level) and how small molecules have been used to probe and modulate signal transduction pathways. The final topic will cover protein translation, stressing mechanistic aspects of protein synthesis and folding in vivo. 555. Molecular Modeling and Simulations. (3 hrs.) Familiarize students with some of the most important computational methods in the molecular sciences. Includes lecture-type presentations of scientific background of methods and computational laboratory using common software packages. 570. [461.] Molecular Physical Chemistry. (3 hrs, Fall & Winter). Prereq: Chem 260 (or 340), Phys 240, and Math 215. Chemistry 461 builds on the introduction to quantum mechanics that was given in Chemistry 260. Students will use the Schrödinger Equation in 1, 2, and 3-dimensions to solve exactly a series of important chemical problems including the harmonic oscillator, the rigid rotor, and the hydrogen atom. Group theory is introduced as an aid for understanding spectroscopic selection rules. Advanced spectroscopy, including transition probabilities, normal vibrational modes, and photoelectron spectroscopies are introduced and then used to deduce molecular structure. The valence-bond and molecular orbital theories of chemical bonding are discussed, and methods for performing quantum chemical calculations, including variational and perturbation methods, are introduced. The quantum mechanics of spin and angular momentum are discussed and used to interpret magnetic resonance spectra. NOTE: Students are strongly encouraged to elect the Computational Chemistry Laboratory (Chemistry 462, 1 credit) in the same term that Chemistry 461 is taken. 575. [463.] Chemical Thermodynamics. (3 hrs, Fall & Winter). Prereq: Chem. 461/462. This is the third of the three-term physical chemistry sequence Chemistry 260/461/463 and builds on material presented in both previous courses. The rigorous mathematical theory of classical thermodynamics will be developed, including applications to entropy, heat engines, solution properties, and phase and chemical equilibria. Modern statistical thermodynamics will be introduced. Modern theories of fundamental reaction rates will be used built on the phenomenological kinetics introduced in Chemistry 260. Methods for determining and understanding solid state structures will be discussed, building on group theory introduced in Chemistry 461. 576. Statistical Mechanics. (3 hrs. Winter) Prereq: Quantum mechanics Constitutes with 571 a full course for students specializing in physical chemistry. The foundation of equilibrium statistical mechanics and applications to problems of chemical interest. Included are discussions of imperfect gases and liquids, mixtures, solids, quantum statistics, surface chemistry and polymers. 580. Molecular Spectra and Structure. (3 hrs, Winter of odd years). Prereq: Chem 570. Review of atomic spectra; rotational, vibration-rotation, and electronic spectra of diatomic and simple polyatomic molecules; and deduction of molecular parameters from spectra. Role of symmetry and representation theory generally. Different spectroscopies from NMR, and EPR through ESCA. 668. Principles of Molecular Symmetry and Solid State Chemistry. (2 hrs, on demand). Prereq: Chem 570 or Solid-State Physics; or permission of instructor. Course will focus on basic surface phenomena which control the physical and chemical properties of extended surfaces. Introductions to a wide range of surface methods and issues regarding metal, semi-conductor and insulator surfaces will be discussed. Fundamental principles regarding the geometric and electronic structure of surfaces, surface thermodynamics, adsorption, desorption processes, surface diffusion, thermally induced surface reactions, state-specific reactions, and ion-surface interactions will be discussed. Current examples will also be discussed to indicate the current state of the art. 1 9 E. F. 670. Principles of Magnetic Resonance. (2 hrs, on demand). Prereq: Chem 570; or permission of instructor. Classical and quantum mechanical treatments of magnetic resonance phenomena. Included will be discussions of spin systems, rotating fields, electron-nucleus interactions, and relaxation phenomena. Experimental and theoretical aspects of nuclear magnetic resonance, electron spin resonance, and Fourier transform NMR; chemical shifts, spin-spin coupling, spin-orbit interactions, hyperfine interactions, spin-lattice relaxation, and other topics. 673. Mechanism and Kinetics. (3 hrs.) Prereq: BC550 or Chem 526/permission. This course will cover enzyme catalytic mechanisms and enzyme kinetics in depth. Ligand binding to macromolecules, transient kinetics, enzyme kinetics, kinetic isotope effects, structure-function analysis, protein structure, enzyme mechanisms and enzyme cofactors will be discussed. An emphasis will be placed on developing the key kinetic and thermodynamic concepts that govern the action of enzymes. 711. Metals in Biology and Medicine. (2 hrs.) MATHEMATICS 404. Intermediate Differential Equations. (3 hrs, Fall & Winter). Prereq: Math 216. This is a course oriented to the solutions and applications of differential equations. Numerical methods and computer graphics are incorporated to varying degrees depending on the instructor. There are relatively few proof s. Some background in linear algebra is strongly recommended. 417. Matrix Algebra I. (3 hrs, Fall & Winter). Prereq: 3 courses beyond Math 110. Many problems in science, engineering, and mathematics are best formulated in terms of matrices --- rectangular arrays of numbers. This course is an introduction to the properties of and operations on matrices with a wide variety of applications. The main emphasis is on concepts and problem-solving, but students are responsible for some of the underlying theory. Diversity rather than depth of applications is stressed. 419. Linear Spaces and Matrix Theory. (3 hrs, Fall & Winter). Prereq: 4 courses beyond Math 110. Math 419 covers much of the same ground as Math 417 (Matrix Algebra I) but presents the material in a somewhat more abstract way in terms of vector spaces and linear transformations instead of matrices. There is a mix of proofs, calculations, and applications with the emphasis depending somewhat on the instructor. A previous proof-oriented course is helpful but by no means necessary. 425. [Stat 425.] Introduction to Probability. (3 hrs, Fall & Winter). Prereq: Math 215 or 285. This course introduces students to useful and interesting ideas of the mathematical theory of probability and to a number of applications of probability to a variety of fields including genetics, economics, geology, business, and engineering. The theory developed together with other mathematical tools such as combinatorics and calculus are applied to everyday problems. Concepts, calculations, and derivations are emphasized. 571. Numerical Methods for Scientific Computing I (3 hrs. Fall & Winter) Prereq: MATH 214, 217, 417, 419, or 513; and one of MATH 450, 451, or 454 Text (required): "Numerical Linear Algebra" by L.N. Trefethen and D. Bau (SIAM) From the preface to the text: "We hope the reader will come to share our view that if any other mathematical topic is as fundamental to the mathematical sciences as calculus and differential equations, it is numerical linear algebra." I hope students in this class will also come to share this view as I have. Math 571 is an introduction to numerical linear algebra, a core subject in scientific computing. Three types of problems are considered: (1) solving a system of linear equations, (2) computing eigenvalues and eigenvectors of a matrix, and (3) least squares problems. These problems often arise in applications in science and engineering, and many algorithms have been developed for their solution. However, standard approaches may fail if the size of the problem becomes large or if the problem is ill-conditioned, e.g. the operation count may be prohibitive or computer roundoff error may ruin the answer. We'll investigate these issues and study some of the accurate, efficient, and stable algorithms that have been devised to overcome these difficulties. The course grade will be based on homework, a midterm exam, and a final exam. Some homework exercises will require computing (Matlab is recommended). Topics: 1. vector and matrix norms, orthogonal matrices, projection matrices, singular value decomposition (SVD); 2. least squares problems, QR factorization, Gram-Schmidt orthogonalization, Householder triangularization, normal equations; 3. stability, condition number, backward error analysis, IEEE floating point arithmetic; 4. direct methods for Ax=b, Gaussian elimination, LU factorization, pivoting, Cholesky factorization; 5. eigenvalues and eigenvectors, Schur factorization, reduction to Hessenberg and tridiagonal form, power method, inverse iteration, shifts, Rayleigh quotient iteration, QR algorithm; 6. iterative methods for Ax=b, Krylov methods, Arnoldi iteration, GMRES, conjugate gradient method, preconditioning; 7. applications: image compression by SVD, least squares data fitting, finite-difference schemes for a two-point boundary value problem, Dirichlet problem for the Laplace equation MOLECULAR, CELL, & DEVELOPMENTAL BIOLOGY 427. Molecular Biology. (4 hrs, Fall & Winter). Prereq: Biol 305, and Biol 310 or 311. Lectures and discussions are concerned with the following topics: the physical methods used in molecular biology; structure and coding capacity of DNA chromosomes; DNA replication; transcription, protein synthesis; prokaryotic and eukaryotic regulation; molecular aspects of development; transposable elements and genetic engineering. 2 0 G. 428. Cell Biology. (4 hrs, Winter). Prereq: Biol 305, and Biol 310 or 311 or Bio.Chem 415. MCDB 428 is designed to provide students with a comprehensive overview of the biology of eukaryotes and prokaryotes at the cellular and molecular level. This course is intended for upper-level undergraduates and graduate students. The information is presented at a level that requires students to integrate information from their other biology, chemistry, and biochemistry courses. The following topics will be included: general techniques used in cell biology; general properties of membranes; secretion, endocytosis, and related processes; organelle biogenesis; signal transduction; lipoprotein metabolism; cytoskeleton and cell motility; cell cycle and its regulation; cell-cell and cell-matrix interactions; programmed cell death; functions of specialized cell types. Students will be expected to integrate the scientific data presented in class as well as to read and interpret basic research drawn from the current scientific literature. Grades will be based on three exams and the discussion section. 514. Topics in Molecular Evolution. (3 hrs, Winter alt). Prereq: Biol 305, and one upper-level course in either molecular or evolutionary biology; and permission of instructor. The subject of this course is methods of comparative DNA/amino acid sequence analysis using an evolutionary approach. Topics of sequence alignment and phylogeny reconstruction using DNA and protein sequences are covered. PHARMACEUTICAL CHEMISTRY 758. H. Methods of Computational Chemistry. (2 hrs, Fall). Prereq: [concurrently] Chem 468; or permission of instructor. A survey of those aspects of computer science most necessary to a practicing researcher in chemistry, particularly pharmaceutical chemistry. Main topics are chemical databases, molecular graphics, and conformational analysis. Two hours lecture a week and some practical exercises. PHYSICS 401. Intermediate Mechanics. (3 hrs, Fall & Winter). Prereq: Phys 126/128 or 240/241, and Math 216. This course is required for physics concentrators. It presents a systematic development of Newtonian mechanics beginning with single particle motion in one dimension and extending through multiparticle systems moving in three dimensions. The conservation laws of energy and linear and angular momentum are emphasized. Lagrangian mechanics is introduced, and Hamiltonian mechanics may be introduced as well. Physical systems treated in detail include the forced damped-oscillator, inverse square forced orbits, harmonic motion in two dimensions, coupled oscillations and rigid body motion in two and three dimensions. Mathematical topics given extensive treatment include vector algebra, elements of vector calculus, ordinary differential equations, plane and spherical polar coordinates and phasors and/or complex numbers. Grades are based on one or two hourly exams and a two-hour final. 405. Intermediate Electricity & Magnetism. (3 hrs, Fall & Winter). Prereq: Phys 126/128 or 240/241, and Math 216. This is a second course on the classical theory of electromagnetism. Familiarity with Maxwell’s equations at the level of 240 is assumed. Physics 340 is strongly recommended. The course elaborates on the theoretical content of the Maxwell theory as well as practical application. Topics: review of vector analysis; electrostatic boundary value problems; magnetostatics; dielectric and magnetic materials; Maxwell’s equations and electrodynamics; the wave equation, electromagnetic waves in free space, waves in conducting and dielectric media; guided waves; electromagnetic radiation, sources of EM radiation. 406. Statistical and Thermal Physics. (3 hrs, Fall & Winter). Prereq: Phys 126/128 or 240/241, and Math 216. An introduction to the thermal and other macroscopic properties of matter, their description in terms of classical thermodynamics, and their microscopic interpretation from the perspective of statistical mechanics. Techniques from classical mechanics, electricity and magnetism, and elementary quantum mechanics will be used. Frequent homework problem assignments, at least one hour exam, and a final examination will be given. 411. Introduction to Computational Physics. (3 hrs, Fall and Winter). Prereq: Phys 401, and Math 216, and some familiarity with a computer language. Introduction to techniques of computational physics with applications in optics, atomic, solid-state, nuclear and particle physics. Topics covered include motion in a force field, calculation of electric and magnetic fields, optical and ion-optical ray tracing, quantum-mechanical (QM) bound states (Schrödinger Equation) and QM barrier penetration and scattering. 417. [Chem 417.] Dynamical Processes in Biophysics. (3 hrs, Winter of even yrs). Prereq: Math 216, and Phys 340 or Chem 463 or 468. Topics include diffusion in biology. (electrical potentials across membranes, nerve action potentials, neuromuscular synapses, the physics of chemoreception, and reaction rate theory); optical techniques. (visible and ultraviolet light absorption, fluorescence and phosphorescence); and random processes in biophysics. (mathematics of random noise, membrane electrical fluctuations, quasielastic light scattering fluctuations, fluorescence fluctuations, and chaotic processes). 453. Quantum Mechanics. (3 hrs, Fall & Winter). Prereq: Phys 390. This course begins with an overview of the experimental and theoretical foundations for quantum mechanics. The theory is developed and applied to simple physical systems, with examples taken from atomic, molecular, condensed matter, nuclear, and particle physics. Topics include: basics of the Schrödinger equations and its solutions in rectangular and spherical coordinates; properties, uses, and interpretations of state functions; expectation values and physical observables; coherence, correlation, and interference. Other topics include spin, the exclusion principle, and some quantum statistical mechanics. 2 1 I. STATISTICS 412. Introduction to Probability and Statistics. (3 hrs, Fall & Winter). Prereq: [concurrently] Math 215 and CS 183. The objectives of this course are to introduce students to the basic ideas of probability and statistical inference and to acquaint students with some important data analytic techniques, such as regression and the analysis of variance. Examples will emphasize applications to the natural sciences and engineering. There will be regular homework, two midterms, and a final exam. 2 2