Parts per million guide - Bio-Link
advertisement

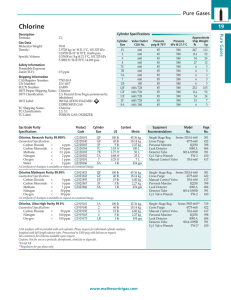
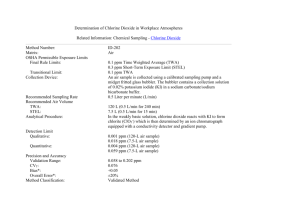
1 PARTS PER MILLION Study guide and self-test “Parts per million” means number of parts of solute per 1 million parts of total solution. 5 ppm chlorine = 5 g. chlorine per 1 million grams solution 5 ounces chlorine per 1 million ounces solution 5 ng. chlorine per 1 million ng. solution BUT: This does not tell you how to make up a solution, because you don’t want to have to weigh your solution to know if you have added enough solvent. SO: To make a solution in the lab, you will want to convert ppm to units like grams / L. Usually, your solvent in the laboratory will be water. Water has a density of 1 gram /mL. THEREFORE: 5 ppm chlorine = 5 g. chlorine per 1 million mL water (solution) = 5 g chlorine / 1000L If we divide the top and bottom by 1 million (106), 5 ppm chlorine = 5 X 10-6 g chlorine = 5 g chlorine 10 –3 L 1 mL solution FOR ANY SOLUTE: 1 ppm in water = 1 g / mL Practice problems: 1. Express a 50 ppm NaCl solution in g/L 2. Express a 330 ppm KCl solution in mg/mL 2 Answers: 1. Express a 50 ppm NaCl solution in g/L 1 ppm = 1 g NaCl/ 1 mL 50 ppm = 50 g/mL Using unit cancellation: 50 g/mL(1g/ 106 g)(1000 mL/L) = 0.05 g/L 2. Express a 330 ppm KCl solution in mg/mL 1 ppm = 1 g KCl/ 1 mL 330 ppm = 330 g/mL Using unit cancellation: 330 g/mL(1 mg/ 103 g) = 0.33 mg/mL