(a) What is the rate law for the reaction?
advertisement

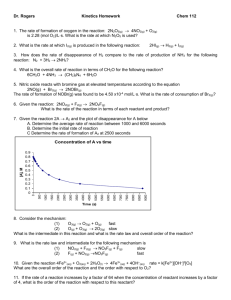
Chapter 14 - Chemical Kinetics 14.1 Introduction - Factors that Affect Reaction Rates • Chemical kinetics is the study of how fast chemical reactions occur. – Generally, the more frequently collisions between reaction particles occur, the faster the reaction. • There are several important factors which affect rates of reactions: Physical state of the reactants – Homogeneous mixtures react faster than heterogeneous mixtures. – When reactants are in different phases: more surface area faster reaction Concentration of the reactants – more reactant molecules so they collide more frequently faster reaction Temperature of the reaction – higher temperature molecules move faster and collide more frequently faster reaction Presence or absence of a catalyst – use a catalyst usually changes mechanism faster reaction 14.2 Reaction Rates • The speed of a reaction is defined as the _________________________________________ It is often determined by measuring the change in concentration of a reactant or product with time [M/sec] You can also use change in moles but if volume is constant [most reactions], then molarity and moles are directly proportional – concentration is easier to directly measure than moles. Pressure is sometimes used for gases. • • The speed of the chemical reaction is its ____________________________________ Suppose A reacts to form B. A B • Let us begin with [A] = 1.00 M • At t = 0 (time zero) there is 1.00 M A and no B present. • At t = 20 sec, there is 0.54 M A and 0.46 M B. • At t = 40 sec, there is 0.30 M A and 0.70 M B. • We can uses this information to find the average rate of the reaction. • For the reaction A B, there are two ways of measuring rate: • The rate of appearance of product B (i.e., change in concentration of B per unit time) as in the preceding example. • The rate of disappearance of reactant A (i.e., the change in concentration of A per unit time). • Note the negative sign! Reaction rates are always positive...the negative indicates a rate of disappearance. Change of Rate with Time • The average rate generally decreases with time • The rate at any instant in time is called the instantaneous rate. • It is the slope of the straight line tangent to the curve at that instant. • Instantaneous rate is different from average rate. • We usually call the "instantaneous rate" the rate. Example: Using the data in table above, calculate the average rate of disappearance of C4H9Cl over the time interval from 50.0 to 150.0 seconds. (b) Using the figure, estimate the instantaneous rate of disappearance of C4H9Cl at t = 0 (the initial rate). a) b) the initial rate is given by the slope of the dashed line (y / x). Pick two points on that line: Reaction Rates and Stoichiometry • For the reaction: C4H9Cl(aq) + H2O(l) C4H9OH(aq) + HCl(aq) • The rate of appearance of C4H9OH must equal the rate of disappearance of C4H9Cl. • What if the stoichiometric relationships are not one-to-one? • For the reaction: 2HI(g) H (g) + I (g) • The rate may be expressed as: 2 2 We can generalize this equation a bit. • For the reaction: aA + bB cC + dD • The rate may be expressed as: o Example: The isomerization of methyl isonitrile CH NC to acetonitrile, CH CN, was studied in the gas phase at 215 C and the following data was obtained: Time (s) 0 2000 5000 8000 12000 15000 [CH NC] (M) 0.0165 0.0110 0.00591 0.00314 0.00137 0.00074 (a) Calculate the average rate of reaction in M/s, for the time interval between each measurement. 3 3 3 Example: Consider the hypothetical reaction: A(aq) B(aq). A flask is charged with 0.065 mol of A and allowed to react to form B in a total volume of 100.0 mL. The following data are collected: Time (min) 0 10 20 30 40 Mol of A 0.065 0.051 0.042 0.036 0.031 (a) Calculate the number of moles of B at each time in the table, assuming there are no molecules of B at time zero (b) Calculate the average rate of disappearance of A for each 10 min interval, in units of M/s. (c) Between t = 10 min and t = 30 min, what is the average rate of appearance of B in units of M/s? Assume the volume of the solution is constant. Example: For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product: (a) H O (g) H (g) + O (g) (b) 2 N O(g) 2 N (g) + O (g) (c) N (g) + 3 H (g) 2 NH (g) 2 2 2 2 2 2 2 2 2 3 Example: Consider the combustion of H (g), 2 H (g) + O (g) 2 H O(g). If hydrogen is burning at the rate of 0.85 mol/s, what is the rate 2 2 2 2 of consumption of oxygen? What is the rate of formation of water vapor? Example: The reaction 2 NO(g) + Cl (g) 2 NOCl(g) is carried out in a closed vessel. If the partial pressure of NO is decreasing at the 2 rate of 23 torr/min, what is the rate of change of the total pressure in the vessel? The change in total pressure is the sum of the changes of each partial pressure. NO and Cl are disappearing and NOCl is appearing. 2 14.3 Concentration and Rate • In general, rates: • Increase _____________________________________________ • Decrease ____________________________________________ • We often examine the effect of concentration on reaction rate by measuring the way in which reaction rate at the beginning of a reaction depends on different starting conditions. Consider the reaction: NH4+(aq) + NO2– (aq) N2(g) + 2H2O(l) • We measure initial reaction rates. • The initial rate is the instantaneous rate at time t = 0 • We find this at various initial concentrations of each reactant. As [NH4+] doubles with [NO2–] constant the ____________________________ • We conclude the rate is proportional to [NH4+]. As [NO2–] doubles with [NH4+] constant the_____________________________ • We conclude that the rate is proportional to [NO2–]. • • The overall concentration dependence of reaction rate is given in a rate law or rate expression. For a general reaction the rate law is: Rate = k [A]m [B]n The proportionality constant k is called the rate constant. Once we have determined the rate law and the rate constant, we can use them to calculate initial reaction rates under any set of initial concentrations. Exponents in the Rate Law For a general reaction with rate law: Rate = k [A]m [B]n The exponents m and n are called reaction orders o The rate is “m” order in A o The rate is “n” order in B We commonly encounter reaction orders of 0, 1 or 2. But, fractional or negative values are possible. The overall reaction order is the sum of the reaction orders. o The overall order of a reaction is m + n + …. Note that reaction orders must be determined experimentally. They DO NOT necessarily correspond to the stoichiometric coefficients in the balanced chemical equation! (but they can and sometimes do) Using Initial Rates to Determine Rate Laws • To determine the rate law, we observe the effect of changing initial concentrations. A reaction is nth order if multiplying the concentration by x causes the rate to multiply by x . n • 1 st For our example – the concentration was multiplied by 2 and the rate was also multiplied by 2 (2 ) so the rate is 1 order with respect to both NH (aq) and NO (aq) + 4 – 2 Most Common Scenarios: Another way to look at order of reaction vs. change in concentration: Zero order rxn: change in concentration = st 1 order rxn: change in concentration = nd 2 order rxn: change in concentration = change in [reactant] change in rate order 0 doubled no change – rate is multiplied by 1 or 2 doubled doubled – rate is multiplied by 2 or 2 doubled quadrupled – rate is multiplied by 4 or 2 tripled no change – rate is multiplied by 1 or 3 tripled tripled – rate is multiplied by 3 or 3 tripled rate is multiplied by 9 or 3 1 2 0 1 2 For the reaction in our example: NH (aq) + NO (aq) N (g) + 2H O(l) The rate law is: Rate = k [NH ] [ NO ] • The reaction is said to be first order in [NH ], first order in [NO ], and second order overall. + 4 – 2 2 2 + 4 – 2 + 4 – 2 Units of Rate Constants • Units of the rate constant depend on the overall reaction order. • For example, for a reaction that is second order overall: • First Order: • Note that the rate, not the rate constant, depends on concentration. • The rate constant IS affected by temperature and by the presence of a catalyst (more on this later). Example: What are the individual and overall reaction orders for the reactions described in the following equations: (1) (2) Reaction (1) 2 N2O5(g) 4 NO2(g) + O2(g) CHCl3(g) + Cl2(g) CCl4(g) + HCl (g) Rate = k [N2O5] Rate = k [CHCl3][Cl2]1/2 Reaction (2) Example: A reaction A + B C, obeys the following rate law: rate = k [B] . (a) if [A] is doubled, how will the rate change? Will the rate 2 constant change? (b) What are the reaction orders for A and B? What is the overall reaction order? (c) What are the units of the rate constant? Example: The decomposition of N O in carbon tetrachloride proceeds as follows: 2 N O 2 NO + O . The rate law is first order in N O . 2 5 2 At 64 C, the rate constant is 4.82 10 s . (a) Write the rate law for the reaction. o -3 -1 5 2 2 2 (b) What is the rate of reaction when [N O ] = 0.0240 M? 2 5 (c) What happens to the rate when the concentration of N O is doubled to 0.0480 M? 2 5 CH Br + OH CH OH + Br . The rate law for this reaction is first order in CH Br and first order -1 Consider the following reaction: -1 3 -1 3 3 -1 in OH . When [CH Br] is 0.0050 M and [OH ] is 0.050 M, the reaction rate at 298 K is 0.0432 M/s. 3 (a) (b) (c) What is the value of the rate constant? What are the units of the rate constant? What would happen to the rate if the concentration of OH were tripled? -1 The iodide ion reacts with hypochlorite ion (the active ingredient in bleach) in the following way: OCl + I OI + Cl . This rapid reaction gives the following rate data: Experiment [OCl ] (M) [I ] (M) Rate (M/s) -1 -1 -1 1 0.0015 0.0015 1.36 10 -4 2 0.0030 0.0015 2.72 10 -4 2.72 10 -4 3 0.0015 0.0030 (a) What is the rate law for the reaction? (b) What is the value of the rate constant? (c) Calculate the rate when [OCl ] = 0.0020 M and [I ] = 0.00050 M -1 -1 5 -1 -1 -1 o Consider the gas-phase reaction between nitric oxide and bromine at 273 C: 2 NO + Br 2 NOBr. The following data for the initial rate of NOBr were obtained: Experiment [NO] (M) [Br ] (M) Initial Rate (M/s) 1 0.10 0.20 24 2 0.25 0.20 150 3 0.10 0.50 60 4 0.35 0.50 735 Determine the rate law. Calculate the value of the rate constant for the appearance of NOBr. How is the rate of appearance of NOBr related to the rate of disappearance of Br ? What is the rate of disappearance of Br when [NO] = 0.075 M and [Br ] = 0.25? 2 2 (a) (b) (c) (d) 2 2 2 -2 8 -1 Consider the reaction of peroxydisulfate ion, S O , with iodide ion, I , in aqueous solution: 2 S O + 3 I 2 SO + I At a particular temperature, the rate of disappearance of S O varies with reactant concentrations in the following manner: Experiment [S O ] (M) [I ] (M) Initial Rate (M/s) -2 2 -1 8 -2 4 -1 3 -2 2 2 -2 8 8 -1 1 0.018 0.036 2.6 10 -6 2 0.027 0.036 3.9 10 -6 3 0.036 0.054 7.8 10 -6 1.4 10 -5 4 0.050 0.072 (a) Determine the rate law for the reaction. Chapter 14 HW Part 1: 14(3, 5, 22, 24, 26, 28, 30, 34, 36) 14.4 The Change of Concentration with Time • Goal: Convert the rate law into a convenient equation that gives concentration as a function of time. First-Order Reactions • Rate must depend on only one reactant and be raised to the 1st power. • For a first-order reaction, the rate doubles as the concentration of a reactant doubles. We know: Rate A k A t After some calculus we get ln A kt ln A t • 0 A ln t A 0 or (slope= −k) kt ln[A]t A plot of ln[A]t versus t is a straight line with slope -k and intercept ln[A] Time (s) 0 Second-Order Reactions • A second-order reaction is one whose rate depends on the reactant concentration to the second power or on the concentration of two reactants, each raised to the first power. • For a 2nd order reaction with just one reactant: 1/[A]t • For a 2nd order reaction with just one reactant: Rate • (slope= k) A 2 k A t (calculus) 1 1 kt At A0 Time (s) A plot of 1/[A]t versus t is a straight line with slope k and intercept 1/[A]0. Half-life • Half-life, t½ , is the time required for the concentration of a reactant to decrease to half its original value. That is, half life, t½, is the time taken for [A]0 to reach ½ [A]0. Mathematically, the half life of a first-order reaction is: A t A 0 ln kt So, for t t1 2 and A 1 2 A t ln • 1 2 A A 0 0 0 kt1 2 Note that the half-life of a first-order reaction is independent of the initial concentration of the reactant. Radioactive decay is 1st order. We can show that the half-life of a second order reaction is: • Note that the half-life of a second-order reaction is dependent on the initial concentration of reactant. Sample 1 The first order rate constant for the decomposition of a certain insecticide in water at 12oC is 1.45 yr-1. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5.0 x 10-7 g/cm3 of water. Assume that the effective temperature of the lake is 12oC. (a) What is the concentration of the insecticide on June 1 of the following year? (b) How long for the conc. of the insecticide to drop to 3.0 x 10-7 g/cm3? (note: conc. units for [A] and [A]0 must be the same) Sample 2 Using the figure to the right, estimate the half-life of the reaction of C4H9Cl with water. Sample 3 The reaction SO2Cl2 SO2 + Cl2 is first order in SO2Cl2. Using the following kinetic data, determine the magnitude of the first order rate constant: Time (s) Pressure SO2Cl2 (atm) 0 1.000 2500 0.947 5000 0.895 7500 0.848 10000 0.803 Sample 4 The following data was obtained for the gas phase decomposition of NO2(g) at 300oC: 2 NO2(g) 2 NO(g) + O2(g) Time (s) [NO2] (M) 0.0 0.01000 50.0 0.00787 100.0 0.00649 200.0 0.00481 300.0 0.00380 (a) What is the rate law for this reaction? (b) what is the value of k (a) From the graphs above, only the plot of 1/[NO2] is a straight line. Thus, the reaction is second order in NO2. (b) From the slope of the straight line graph, we can get: Sample 5 The decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 10-2 s-1. (a) if we begin with an initial SO2Cl2 pressure of 375 torr, what is the pressure of this substance after 65 s? (b) at what time will the pressure of SO2Cl2 decline to one-tenth its initial value? Consider the data presented (a) By using appropriate graphs, determine whether the reaction is first or second order. (b) What is the value of the rate constant for the reaction? (c) What is the half life for the reaction? (a) make both first and second order plots to see which is linear (b) the slope of the line in the graph of 1/[A] vs time is the rate constant (c) use the half-life formula for a 2nd order reaction: HW #2 14( 40, 44, 46, 48, 52) 14.5 Temperature and Rate Most reactions speed up as temperature increases The rate law has no temperature term in it, so the rate constant (k) must depend on temperature. – We see an approximate doubling of the rate with each 10oC increase in temperature. • • The Collision Model • Rates of reactions are affected by ___________________________________. • An explanation is provided by the collision model, based on kinetic-molecular theory. • In order for molecules to react they must collide. • The ____________________________________________________________________ - The more molecules present, the greater the probability of collision faster rate - The higher the temperature, the faster molecules move and more often they collide faster rate However, not all collisions lead to products. • In fact, only a small fraction of collisions lead to products. • In order for a reaction to occur the reactant molecules must collide in the ___________________ AND ___________________________________ to form products. The Orientation Factor The orientation of a molecule during collision can have a profound effect on whether or not a reaction occurs. • Consider the reaction between Cl and NOCl: • If Cl collides with Cl of NOCl, the products are Cl2 and NO. • If the Cl collides with the O of NOCl, no products are formed. Activation Energy Arrhenius: molecules must posses a minimum amount of energy to react. In order to form products, bonds must be broken in the reactants. • Bond breakage requires energy. (ΔHrxn = bonds broken – bonds formed) • Molecules moving too slowly, with too little kinetic energy, don’t react when they collide. • • Activation energy, Ea ____________________________________________________________________________ (varies with the reaction). Energy is required to stretch and break the bond between the CH3 group and the NC group • The species at the top of the barrier is called the activated complex or transition state. • Energy is released when the C-C bond is formed. • The change in energy for the reaction is the difference in energy between CH3NC and CH3CN. • The activation energy is the difference in energy between reactants, (CH3NC) and the transition state. • The rate depends on the magnitude of the Ea. • In general, _____________________________________________________________. • Notice that if a forward reaction is exothermic (CH3NC CH3CN), then the reverse reaction is endothermic (CH3CN CH3NC). How does this relate to temperature? • At any particular temperature, the molecules present have an average kinetic energy. – Some molecules have less energy than the average while others have more than the average value. – Molecules that have an energy equal to or greater than Ea have sufficient energy to react. • The fraction of molecules with an energy equal to or greater than Ea is given by: (R is 8.314 J/mol-k , Temp in K, Ea in J) At a higher temperature, more molecules have the minimum Ea needed to react and the rate is faster. The Arrhenius Equation • Arrhenius discovered that most reaction-rate data obeyed an equation based on three factors 1. The number of collisions per unit time. 2. The fraction of collisions that occur with the correct orientation. 3. The fraction of the colliding molecules that have an energy equal to or greater than Ea. Arrhenius equation: • • Where k is the rate constant, Ea is the activation energy(J), R is the ideal-gas constant (8.314 J/K•mol) and T is the temperature (K). A is the frequency factor. – It is related to the frequency of collisions and the probability that a collision will have a favorable orientation. More Useful Versions of the Arrhenius Equation ln A slope = -Ea / R ln k • A graph of ln k vs 1/T will be a line with slope of –Ea/R and a y-intercept of ln A. • Useful when we have 2 different k values at 2 different temperatures 1/T Example1: Consider a series of reactions having the following energy profiles: Assuming that all three reactions have nearly the same frequency factors, rank the reactions from slowest to fastest. Example 2: Calculate the fraction of atoms in a sample of argon gas at 400 K that have an activation energy of 10.0 kJ or greater. Example 3: The gas phase reaction Cl(g) + HBr(g) HCl(g) + Br(g) has an overall enthalphy change of - 66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction and label Ea and ΔE. (b) What is the activation energy for the reverse reaction? Example 4: A certain first order reaction has a rate constant of 2.75 10-2 s-1 at 20oC. What is the value of k at 60oC if Ea = 75.5 kJ/mol Example 5: The rate of the reaction CH3COOC2H5 + OH-1 CH3COO-1 + C2H5OH was measured at several temperatures and the following data were collected. Using the data, construct a graph of ln k versus 1/T and determine the value of Ea Example 6: The activation energy of a certain reaction is 65.7 kJ/mol. How many times faster will the reaction occur at 50oC rather then 0oC assuming equal initial concentrations? Since we are considering one reaction and temp only affects k which directly affects rate…the ratio of k values equals the ratio of rates at the two temperatures. 14.6 Reaction Mechanisms • A chemical equation provides information about substances present before and after a reaction. • The reaction mechanism is the process of how the reactants become products. • Mechanisms provide a picture of which bonds are broken and formed during the course of a reaction. • Most reactions occur as a series of small steps or changes. Elementary steps are processes that occur in a single step. • The number of molecules present in an elementary step is the molecularity of that elementary step. Unimolecular: one molecule in the elementary step. Bimolecular: two molecules in the elementary step. Termolecular: three molecules in the elementary step Multistep Mechanisms • consist of a sequence of elementary steps. – The elementary steps must add together to give the balanced chemical equation. • Intermediates - these are species that appear in an elementary step but are neither a reactant nor product. – They are formed in one elementary step and consumed in another. – They are NOT found in the balanced equation for the overall reaction. Rate Laws for Elementary Steps • The rate laws of the elementary steps determine the overall rate law of the reaction. • For elementary processes, we DO NOT need to find the reaction order by experimentation – it matches the stoichiometry of the equation • The rate law of an elementary step is determined by its molecularity. Unimolecular processes are first order. Bimolecular processes are second order. Termolecular processes are third order. Rate Laws for Multistep Mechanisms • When a reaction occurs by mechanisms with more than one step, the slowest step limits the overall reaction rate. – This is called the rate-determining step of the reaction. – This step governs the overall rate law for the overall reaction. Example: Step 1: A + A A2 Step 2: A2 + B A2B Step 3: A2B + B A2B2 • The rate law includes reactant molecules (not intermediates) that react during and before the r-d-s (slow step). If step 1 is the slowest: _________________________________ If step 2 is the slowest: ________________________________ If step 3 is the slowest: _________________________________ Consider the reaction: NO2(g) + CO(g) → NO(g) + CO2(g) Here is a proposed a mechanism for the reaction: Step 1: NO2(g) + NO2(g) NO3(g) + NO(g) slow step • • • • • • • Step 2: NO3(g) + CO(g) NO2(g) + CO2(g) fast step (Note that NO3 is an intermediate.) The overall reaction rate will depend on the slowest step Rate = k[NO2]2 If we do an experiment and find that the experimentally derived rate law is: Rate = k[NO2]2 Then our theoretical rate law is in agreement with the experimental rate law. This supports (but does not prove) our mechanism. 14.7 Catalysis A catalyst is a substance that changes the speed of a chemical reaction without any permanent change to itself – Works by providing an alternate mechanism for the reaction – one that has a lower activation energy • Lowering the activation energy allows for more molecules with Ea large enough to react – more collisions that can make products – higher rxn rate (without changing temperature) – Very common in nature – Much research to find new catalysts AND, much research to find “inhibitors”(substances that slow down an undesired reaction) Example: corrosion Homogeneous catalyst – _________________________________________________ Heterogeneous catalyst – _________________________________________________ Enzymes – ____________________________________________________________ Effect of a catalyst on a reaction A catalyst provides an alternate mechanism that has a lower Ea than the original mechanism – this allows the reaction to occur at a faster rate Example 7: What is the molecularity of each of the following elementary processes? Write the rate law for each. (a) Cl2 2 Cl (b) OCl-1 + H2O HOCl + OH-1 (c) NO + Cl2 NOCl2 Example 8: Based on the following reaction profile, (a)how many intermediates are formed in the reaction A D? (b)How many transition states are there? (c)Which step is the fastest? (d) Is the reaction A D exothermic or endothermic? Example 9: The following mechanism has been proposed for the gas-phase reaction of H2 with ICl: H2 + ICl HI + HCl HI + ICl I2 + HCl (a)Write the balanced equation for the overall reaction (b)Identify any intermediates (c)Write rate laws for each elementary reaction in the mechanism (d)If the first step is slow and the second one is fast, what rate law do you expect to be observed for the overall reaction? (e) If the second step is the rds, what rate law would you expect for the reaction The reaction 2 NO + Cl2 2 NOCl obeys the rate law: rate = k [NO]2 [Cl2]. The following mechanism has been proposed for this reaction: NO + Cl2 NOCl2 NOCl2 + NO 2 NOCl (a) What would the rate law be if the first step were rate determining? (b) Based on the observed rate law, what can we conclude about the relative rate of the the two steps? The oxidation of SO2 to SO3 is catalyzed by NO2. The reaction proceeds as follows: NO2(g) + SO2(g) NO(g) + SO3(g) 2 NO(g) + O2(g) 2 NO2(g) (a) Show that the two reactions can be summed to give the overall oxidation of SO2 by O2 to give SO3. Hint: the top reaction must be multiplied by a factor so the NO and NO2 cancel out. (b) Why do we consider NO2 a catalyst and not an intermediate in this reaction? (c) Is this an example of homogeneous catalysis or heterogeneous catalysis? HW: 14(54, 58, 61, 64, 66, 74, 78, 80)