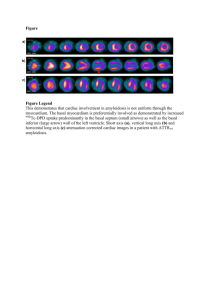
Infiltrative diseases Anderson-Fabry`s disease AFD disease is
advertisement

Infiltrative diseases Anderson-Fabry’s disease AFD disease is caused by an X-linked deficiency of lysosomal α-galactosidase A. It has been reported in up to 6% of men and 12% of women with late-onset HCM1. Lysosomal α-galactosidase A deficiency results in multiorgan damage determined by glycosphingolipid deposition2. In the heart, glycosphingolipid deposition is accompanied by secondary changes, such as myocyte hypertrophy, which can mimic the morphological and clinical picture of HCM3. Chest pain is a common clinical symptom in patients with AFD, being reported in up to 60% of homozygous males and heterozygous females with or without significant LVH. It is often associated with electrocardiographic ST-segment and T wave abnormalities and with severely impaired CFR at PET imaging and is caused by accumulation of glycosphingolipids in endothelial cells and SMC4. Progressive deterioration of LV function and myocardial scarring occurs in a significant number of patients5. Accordingly, growing evidence suggests that LGE may identify areas of myocardial damage. In particular, one study showed that patients with AFD disease–related LVH exhibit LV LGE with a typical pattern, characterized by the involvement of the infero-lateral basal or mid basal segments and a by a mesocardial distribution6. A timely diagnosis of AFD has relevant therapeutic implications because enzyme replacement and enzyme enhancement therapy have been revealed to be effective in treating the disease. This treatable disease, however, is frequently undiagnosed. Measurement of α-galactosidase A activity in peripheral blood in patients with LVH has been proposed to detect patients with AFD; however, this assessment may be unreliable in female carriers and in male patients with specific gene mutations, in whom the nearly normal enzymatic activity and the lack of systemic manifestations, including angiokeratomas, make its identification more difficult7. Unfortunately, clinical characteristics and ECG findings do not provide a reliable and definite diagnosis of AFD. In AFD enzyme replacement treatment with alpha galactosidase A reduces left ventricular mass and improves cardiac function as well as clinical outcome, while it does not seem to improve CMD8. It remains to establish whether a longer period of replacement therapy may lead to improvement of CMD. Amyloidosis Cardiac amyloidosis is classified by the protein precursor as primary, secondary (reactive), senile systemic, hereditary, isolated atrial, and hemodialysis-associated amyloidosis. These distinct forms are differentiated by means of immuno-histochemical and genetic testing, and prognosis and therapeutic strategies differ among these subtypes9. A sizeable proportion of patients with primary cardiac amyloidosis report typical chest pain caused by CMD. Indeed, amyloid deposits typically spare the epicardial vessels, while involvement of the intramural vasculature is present in 90% of patients with primary cardiac amyloidosis although it is much less frequent in senile amyloidosis 10. Vascular deposition of amyloid first occurs in the media but, with disease progression, it involves the adventitia and intima. Although severe vascular obstruction is rare, occurring in about 5% of cases, diffuse involvement leads to numerous endocardial foci of ischemia, microinfarctions, and eventual fibrosis, further contributing to myocardial dysfunction and, in case of involvement of the conduction system, cardiac electrical disorders. Clinical studies have confirmed a reduction of CFR in patients with primary amyloidosis and angina noninvasively using contrast echocardiography11 and invasively using a Doppler wire12. Treatment of amyloidosis depends on the type of the disease. To the best of our knowledge no study has hitherto assessed the effects of specific or nonspecific forms of therapy on CMD in amyloidosis. Treatment of iatrogenic CMD In the setting of PCI for obstructed saphenous vein grafts mechanical prevention of distal embolization by filters13 or proximal protection devices14 has been demonstrated to reduce the occurrence of peri-procedural myocardial infarction and of major cardiac events. With regard to pharmacological treatment, a loading dose of aspirin15 and clopidogrel16, in patients already on treatment, has been found to reduce peri-procedural myocardial infarction. GPIIb/IIIa inhibitors also have been shown to reduce peri-procedural infarction in several clinical trials, a finding confirmed in a meta-analysis17. However, in the era of double platelet antiaggregation, GPIIb/IIIa inhibitors are recommended only in high risk patients with intracoronary thrombus18. Importantly, administration of statins has shown to reduce peri-procedural infarction both during elective19,20,21,22 and urgent PCI. A meta-analysis23 confirmed that statin administration prior to PCI almost halves the rate of peri-procedural myocardial infarction. More recently an atorvastatin load (120 mg) before PCI was shown to reduce post-procedural CK-MB and TnI elevation in patients with non ST elevation ACS who were on previous statin therapy24, while this effect was not observed in patients with stable angina. With regard to the treatment of functional CMD alterations, Rimoldi et al. using PET demonstrated in a randomized, double blind, placebo controlled trial that oral pre-treatment with the 1-adrenergic antagonist doxazosin restored CFR immediately after PCI in patients treated with the active drug, but not in controls25. In the setting of CABG ischemic preconditioning was the first ‘conditioning’ strategy applied in man in the attempt to reduce myocardial damage26. In a recent meta-analysis preconditioning has been found to be associated with fewer ventricular arrhythmias and need for inotropic support in the absence, however, of any benefit on clinical end-points27. Remote preconditioning also has been assessed in one study and was found to reduce peri-operative myocardial injury measured by the 72-hour area-under-the-curve of serum TnT concentrations28. Subsequent studies, however, gave discordant results29,30. With regard to pharmacological treatment, adenosine has been shown in several clinical studies to reduce peri-operative myocardial injury and improve cardiac indices when administered either as an intravenous therapy or when added to the cardioplegic solution31. However, the results of these studies are rather conflicting32. Volatile anesthetic agents have been shown to induce conditioning in experimental studies33. A meta-analysis compared volatile vs intravenous anesthetics: patients who received volatile anesthetic agents exhibited better cardiac function, less requirement for inotropic response, less peri-operative myocardial injury, as assessed by serum TnI levels, and a shorter duration of mechanical ventilation and hospital stay. However, no differences were observed in the incidence of infarction, intensive care unit stay and in-hospital mortality. Other meta-analyses confirmed these data34. Finally, in a recent meta-analysis pre-operative or early post-operative statin administration was found to be associated with a lower rate of all-cause mortality, atrial fibrillation, and stroke in the absence of any beneficial effect on post-operative infarction or renal failure35. Thus, in the setting of both PCI and CABG statins appear to be beneficial, probably through improvement of CMD related to their pleiotropic effects. References 1 Chimenti C, Morgante E, Tanzilli G, et al. Angina in fabry disease reflects coronary small vessel disease. Circ Heart Fail 2008;1:161-9. 2 Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill, 2001:3733-74. 3 Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med 1995;333:288-93. 4 Elliott PM, Kindler H, Shah JS, et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 2006;92:357-360. 5 Shah JS, Lee P, Hughes D, et al. The natural history of left ventricular systolic function in Anderson-Fabry disease. Heart 2005;91:533-534. 6 De Cobelli F, Esposito A, Belloni E, et al. Delayed-enhanced cardiac MRI for differentiation of Fabry's disease from symmetric hypertrophic cardiomyopathy. AJR Am J Roentgenol 2009;192:W97-102. 7 Chimenti C, Pieroni M, Morgante E, et al. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 2004; 110:1047-1053. 8 Kalliokoski RJ, Kantola I, Kalliokoski KK, Engblom E, Sundell J, Hannukainen JC, Janatuinen T, Raitakari OT, Knuuti J, Penttinen M, Viikari J, Nuutila P. The effect of 12-month enzyme replacement therapy on myocardial perfusion in patients with Fabry disease. J Inherit Metab Dis 2006;29:112-118. 9 Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: a comprehensive review. Arch Intern Med 2006;166:1805-1813. 10 Crotty TB, Li CY, Edwards WD, Suman VJ. Amyloidosis and endomyocardial biopsy: correlation of extent and pattern of deposition with amyloid immunophenotype in 100 cases. Cardiovasc Pathol 1995;4:39-42. 11 Abdelmoneim SS, Bernier M, Bellavia D, Syed IS, Mankad SV, Chandrasekaran K, Pellikka PA, Mulvagh SL. Myocardial contrast echocardiography in biopsy-proven primary cardiac amyloidosis. Eur J Echocardiogr 2008;9:338-341. 12 Al Suwaidi J, Velianou JL, Gertz MA, Cannon RO 3rd, Higano ST, Holmes DR Jr, Lerman A. Systemic amyloidosis presenting with angina pectoris. Ann Intern Med 1999;131:838-841. 13 Baim DS, Wahr D, George B, Leon MB, Greenberg J, Cutlip DE, Kaya U, Popma JJ, Ho KK, Kuntz RE; Saphenous vein graft Angioplasty Free of Emboli Randomized (SAFER) Trial Investigators. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation 2002;105:1285-1290. 14 Mauri L, Cox D, Hermiller J, Massaro J, Wahr J, Tay SW, Jonas M, Popma JJ, Pavliska J, Wahr D, Rogers C. The PROXIMAL trial: proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: a randomized, prospective, multicenter clinical trial. J Am Coll Cardiol 2007;50:1442-1449. 15 King SB III, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO; 2005 WRITING COMMITTEE MEMBERS, Feldman TE, Kern MJ, O'Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2008;117:261-295. 16 Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005;111:2099-2106. 17 Labinaz M, Ho C, Banerjee S, Martin J, Chen S, Mensinkai S. Meta-analysis of clinical efficacy and bleeding risk with intravenous glycoprotein IIb/IIIa antagonists for percutaneous coronary intervention. Can J Cardiol 2007;23:963-970. 18 Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D; ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:29993054. 19 Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of Myocardial Damage during Angioplasty) study. Circulation 2004;110:674-678. 20 Briguori C, Visconti G, Focaccio A, Golia B, Chieffo A, Castelli A, Mussardo M, Montorfano M, Ricciardelli B, Colombo A. Novel Approaches for Preventing or Limiting Events (Naples) II Trial Impact of a Single High Loading Dose of Atorvastatin on Periprocedural Myocardial Infarction. J Am Coll Cardiol 2009;54:2157-2163. 21 Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G, Montinaro A, Di Sciascio G. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 2007;49:1272-1278. 22 Yun KH, Jeong MH, Oh SK, Rhee SJ, Park EM, Lee EM, Yoo NJ, Kim NH, Ahn YK, Jeong JW. The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol 2009;137:246-251. 23 Merla R, Reddy NK, Wang FW, Uretsky BF, Barbagelata A, Birnbaum Y. Meta-analysis of published reports on the effect of statin treatment before percutaneous coronary intervention on periprocedural myonecrosis. Am J Cardiol 2007;100:770-776. 24 Di Sciascio G, Patti G, Pasceri V, Gaspardone A, Colonna G, Montinaro A. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol 2009;54:558-565. 25 Rimoldi O, Spyrou N, Foale R, Hackett DR, Gregorini L, Camici PG: Limitation of coronary reserve after successful angioplasty is prevented by oral pretreatment with an 1-adrenergic antagonist. J Cardiovasc Pharmacol 2000; 36: 310-315. 26 Yellon DM, Alkhulaifi AM, Pugsley WB. Preconditioning the human myocardium. Lancet 1993;342:276-277. 27 Walsh SR, Tang TY, Kullar P, Jenkins DP, Dutka DP, Gaunt ME. Ischaemic preconditioning during cardiac surgery: systematic review and metanalysis of perioperative outcomes in randomized clinical trials. Eur J Cardiothorac Surg 2008;34:985-994. 28 Hausenloy DJ, Mwamure PK, Venugopal V Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ,Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575-579. 29 Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G.. Remote ischaemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 2010;105:657-664. 30 Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, John L, Shah AM, Marber MS, Kunst G. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic Res Cardiol 2011;106:511-519. 31 Mentzer RM Jr, Rahko PS, Molina-Viamonte V, Canver CC, Chopra PS, Love RB, Cook TD, Hegge JO, Lasley RD. Safety, tolerance, and efficacy of adenosine as an additive to blood cardioplegia in humans during coronary artery bypass surgery. Am J Cardiol 1997;79:38-43. 32 Belhomme D, Peynet J, Florens E, Tibourtine O, Kitakaze M, Menasche P. Is adenosine preconditioning truly cardioprotective in coronary artery bypass surgery? Ann Thorac Surg 2000;70:590-594. 33 Kato R, Foex P. Myocardial protection by anesthetic agents against ischemia–reperfusion injury: an update for anesthesiologists. Can J Anaesth 2002;49:777–791. 34 Landoni G, Biondi-Zoccai GG, Zangrillo A Bignami E, Scandroglio AM, Dedola E, De Luca M, Tritapepe L, Crescenzi G, Zangrillo A.. Desflurane and sevoflurane in cardiac surgery: a metaanalysis of randomized clinical trials. J Cardiothorac Vasc Anesth 2007;21:502–511. 35 Liakopoulos OJ, Choi YH, Haldenwang PL, Strauch J, Wittwer T, Dörge H, Stamm C, Wassmer G, Wahlers T. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J 2008;29:1548-1559.