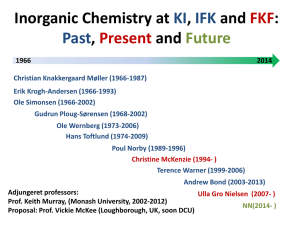
UNIVERSITY OF NAIROBI Course Title: General and Inorganic
advertisement

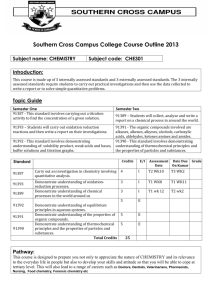
UNIVERSITY OF NAIROBI Course Title: General and Inorganic Chemistry (Chemistry 1) Course code: FME151; FCE 181 and FBE105 Introduction: This is a general chemistry course offered to engineering students to boost their understanding of chemistry concepts which are key building blocks for engineering courses such as material science. The course covers selected topics in Inorganic, Analytical, Environmental and Physical Chemistry. Programme: Engineering Lecture time: Monday 8-10 am; Tuesday 11-1 pm Practicals: Wednesday 8-11 am; Thursday 10-1 pm Lecture Venue: Monday 8-10 am Main Campus Room E001; Tuesday 11-1 pm CELT 201 Practicals Venue: Chiromo Campus, Department of Chemistry Labs Pre-requisite where appropriate: Student must have met the minimum qualification for engineering programme at the University of Nairobi Office location: Chemistry Dept. Room 114 Consultation Hours: Thursday 4-5 pm; Friday3-5 pm Course objectives 1) To provide fundamental concepts of Inorganic and Physical chemistry to engineering students 2) To provide practical concepts of analytical chemistry to engineering students Detailed Course Content Course outline 1) 2) 3) 4) 5) 6) 7) Atomic structure Chemical bonding Chemical kinetics Chemical equilibrium Solutions Adsorption Environmental pollution. Course content Atomic structure: Introduction to atomic structure; electromagnetic radiation, Borh’s atomic model; wave mechanical model of atomic structure; electron configuration, effective nuclear charge. Chemical bonding: ionic bond, covalent bond, metallic bond, hydrogen bond. Chemical kinetics: Rate of reactions and rate equations; factors that influence rate of reaction, order of reaction, molecularity of reaction, activation energy, Chemical equilibrium: Equilibrium law, Equilibrium constant, homogeneous equilibrium, heterogeneous equilibrium, Lechatelier’s principle, chemical transformations and energy changes. Solutions: types of solutions, Henry’s law, solubility and solubility curves, ionic equilibria. Adsorption: types of adsorption, adsorption isotherms, application of adsorption. Environmental pollution: Environmental segments, types of pollutants, air pollution, acid rain, smog formation, green house effect, ozone layer, water treatment. Instructional methods: 4 hour lectures per week, 6 hour practicals per week; tutorials questions Course materials Text books 1) Satya P., Tuli, G.D, Basu, S.K., Madan R.D., (2004). Advanced Inorganic Chemistry Volume 1. S. Chand & Company ltd. Ram Nagar, New Delhi-110055. 2) Neetu G. AND Sanjay Kumar, (2005) Concise Engineering Chemistry (Theory and Practicals) AITBS publishers & distributors. Krishan Nagar, Delhi-11005, India. 3) Negi A.S. and Anand S.C., (2003) A textbook of Physical Chemistry. New Age International (P) Ltd, publishers. New Delhi. 4) SawyerC.N, MacCarty, P., Parkin, G. F., (2003) Chemistry for Environmental engineering and Science. TATA McMcGraw-Hill publishing Company Limited New Delhi. 5) Chauhan B.S. (2008) Engineering chemistry. Laxmi publications (P) ltd. 2 nd Ed. 113, Golden House, Daraganj, New Delhi-110002. 6) Dara S.S., (2005) a Text book of Engineering Chemistry. S. Chand and Company ltd. Ram Nagar, New Delhi -110055. 7) Deeper Sharma, (2007) Engineering Chemistry. Paragon International Publishers. New Delhi-110002, India. Evaluation: CAT 30%, Exam 70% Contacts Lecturer: Mr. Vincent O. Madadi Address: Department of Chemistry, University of Nairobi, P. O. Box 30197-00100, Nairobi, Kenya Tel: 4446138 ext 2185 Cell phone: 0720742415 Email: vmadadi@uonbi.ac.ke, madadivin2002@yahoo.com Website: http://www.uonbi.ac.ke/staff/vmadadi