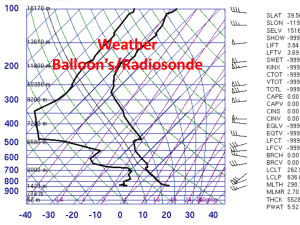
Water and Energy
advertisement

Energy & The Water Cycle Activity 3: Cooling and Heating Water Observe differences in cooling and heating of fresh and salt water in balloons. Materials A. Ring apparatus and Thermometer-Balloon assembly: Ring stand and attachment 2 thermometers (or probes) with holes or other attachment points at top (must be same make and model and reading same temperature) 2 small balloons (e.g. water balloons) of different colors Fresh water Salt water 2 rubber bands Syringe 2 paper clips B and C. To cool water (B) and then heat to room temperature (C): Crushed ice Salt (we recommend rock or sea salt) Fresh water 1 Stirring rod 2 250-mL beakers Stopwatch D. To boil water: Safety goggles Hot plate 1 Stirring rod Ruler Salt water 1 250mL beaker Stopwatch Procedure A. Ring apparatus and Thermometer-Balloon assembly: 1. Look at one of your thermometers and record the temperature of the room. Also check to see if the other thermometer is reading the same temperature (let me know if they’re not). You will need this information in step C. 2. Fill a syringe with fresh water. Inject a small balloon with the water until it is full but not stretched. 3. Carefully insert the bulb of a thermometer into the bulb of the balloon. The bottom of the thermometer should not touch bottom of the balloon. 4. Fasten the balloon onto the thermometer with a rubber band. There should be no air in the balloon. 5. Record which color balloon has fresh water. 6. Repeat a-d for salt water. It is important that your salt-water balloon be attached to the thermometer the same way as your fresh water balloon. When you hold up the two thermometer-balloon assemblies, they should look alike except for the balloon color. 7. Use paper clips or pipe cleaners to hang your two thermometers close to each other (both bulbs should be at the same height). B. To cool water: (Do NOT record data in the chart for this part) Write a prediction on which balloon will freeze first here: 1. Create a cold water bath of crushed ice, salt, and tap water. a. Fill a 250mL beaker three-quarters full of crushed ice. b. Add approximately one-fourth cup of salt. c. Add some water to the beaker, so that the mixture is mostly ice. You do not want to add too much water so that the ice melts. Stir components well. 2. Put your slushy mixture under the ring stand and attach your thermometers to the ring so they hang into the ice bath but do not touch the bottom of the beaker (see Fig. 5-17). 3. Stir your ice bath around your thermometers. Avoid touching the thermometers as you stir. a. Continue to stir until you notice a phase change in one or both balloons (use your stirring rod to gently touch the balloons starting at 5min and use the firmness of the balloons as an indicator of a phase change it’s going to freeze and harden). b. Note the temperature of both balloons when you detect a phase change in one of the balloons. Record your observations about the firmness of each balloon. c. Keep your balloons submerged in the ice bath until instructed. Fig. 5-17. Apparatus with thermometer-balloon assembly for cooling and warming water. C. To heat water to room temperature: 1. Fill a 250mL beaker with room temperature tap water. 2. Predict which type of water will reach room temperature first. Write down your prediction. 3. Gently remove the thermometers from the ice bath and place into the beaker of room temperature water. a. Do this by rotating and lifting the thermometers out of the ice bath breaker and removing the beaker. The ring apparatus and thermometer-balloon assembly does not have to move. b. Place the beaker with room temperature water where the ice bath beaker used to be. Gently place the thermometers into the water. 4. Record the temperature every 20 seconds for each balloon as the water warms to room temperature (Table 5-2). Stir gently the water around your thermometers. Avoid touching the thermometers as you stir. You may not have to fill out the whole table; stop when both of your balloons reach room temperature. Table 5-2. Table of time and temperatures of fresh and salt water as they warm D. To boil water: 1. Predict which water will boil first. Write down your prediction. 2. Place a 250-mL beaker on a hot plate under the ring attachment of your ring stand. Make sure that the bottom of the ring stand is not under the hot plate so you can easily lift the ring stand away. 3. Adjust the height of the thermometers on their pipe cleaners around the ring attachment so they are 1cm from the bottom of the beaker. Add enough salt water (approximately 150 mL) to the beaker to cover the stems of the balloons. The balloons should be entirely submerged in the beaker water, but just enough to cover the tops of the balloons. 4. Put on your safety goggles. Turn the hot plate on to “high”. Stir the water bath continually until this experiment is completed. Avoid touching the thermometers as you stir. 5. Record the temperature of each balloon every 2 minutes (Table 5-3). Watch your balloons very closely as the temperature approaches 100˚C. If your balloons expand quickly, remove them from the water by picking up the ring stand set-up. You may not have to fill out the whole of Table 5-3; you can stop either when your balloons need to be removed from the water or remain the same temperature for more than two measurements. Table 5-3. Table of time and temperatures of fresh and salt water as they boil Questions 1. Was there any difference in the way the balloons in the ice water bath felt after 5 minutes? 2. What kind of bonds form between water molecules when water freezes? 2B. What do you think salt does to the bonds when water freezes? 3. Which water started to heat faster from being cold to room temperature? Why the difference? 4. Which boiled first, the fresh water or the salt water? Was there any difference in the temperatures at which they boiled? Explain your observations. 5. Combine your data from both warming and boiling water to create a graph. Place temperature on the y-axis and time on the x-axis. Put your data from both fresh and salt water on your graph. 6. Were there plateaus (flat areas) on your graphs? Did it correlate with a phase change? 6A. Explain how this relates to the making and breaking of H bonds between water molecules. 7. How would these plateaus that water has, help to moderate temperatures on Earth? 8. If planet A had lots of water on it and planet B had little water on it, how would they differ as both planets started to heat up? 9. How does salt affect the melting and boiling points of water? 10. Why do chefs add salt to water before boiling when cooking vegetables or pasta? 11. Why did we have to add salt to the cooling and heating water baths?