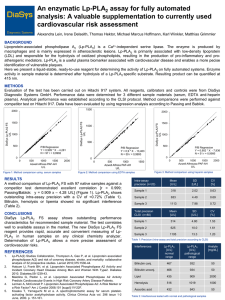
EURHEARTJ-D-11-02896R3 SUPPLEMENTARY ONLINE

EURHEARTJ-D-11-02896R3
SUPPLEMENTARY ONLINE MATERIAL
METHODS
Study Population
PIVUS sample
Eligible were all subjects aged 70 living in the community of Uppsala, Sweden. The subjects were randomly chosen from the register of community living. 1016 subjects participated giving a participation rate of 50.1%. Of those, a random of 306 subjects was evaluated with
MR-angiography. The study was approved by the Ethics Committee of the University of
Uppsala.
All subjects were investigated in the morning after an over-night fast. No medication or smoking was allowed after midnight. The participants were asked to answer a questionnaire about their medical history, smoking and exercise habits and regular medication. Blood pressure was measured by a calibrated mercury sphygmomanometer to nearest mmHg after at least 30 min of rest and the average of three recordings was used. Lipid variables and fasting blood glucose were measured by standard laboratory techniques. High sensitive CRP was measured in human serum by an ultrasensitive particle enhanced immunoturbidimetric assay
(Orion Diagnostica, Espoo, Finland). Glomerular filtration rate (GFR) was calculated by the
Modification of Diet in Renal Disease (MDRD) formula (http://www.medcalc.com/gfr.html).
Daily intakes of energy and alcohol were given by a 7 days dietary recording 1 . Exercise habits were given at a four-graded scale, where 0 was less than 2 times of light exercise a week, 1 denoted more than 1 occasion of light exercise a week, 2 was 1-2 times of heavy exercise and 3 was heavy exercise more than 2 times a week.
Approximately 10% of the cohort reported a history of coronary heart disease, 4% reported stroke, 3% heart failure, and 9% diabetes mellitus. Almost half the cohort reported any cardiovascular medication (45%), with antihypertensive medication being the most prevalent
(32%). Fifteen percent reported use of statins, while insulin and oral antiglycemic drugs were reported in 2 and 6%, respectively (see reference
2
for details).
The FAST-MI sample
The population and methods of the FAST-MI (French registry of Acute ST-elevation and non-ST-elevation Myocardial Infarction) registry have been described in detail elsewhere
3
.
EURHEARTJ-D-11-02896R3
Briefly, the objective of the FAST-MI registry was to gather complete and representative data on the management and outcome of patients admitted to intensive care units for definite AMI, irrespective of the type of institution to which they were admitted (i.e., university hospitals, public hospitals or private clinics). All consecutive adult patients (≥18 years) were included in the registry if they had elevated serum markers of myocardial necrosis higher than twice the upper limit of normal for creatine kinase, creatine kinase-MB or elevated troponins, and either symptoms compatible with AMI and/or electrocardiographic changes on at least two contiguous leads with pathologic Q waves (≥0.04 sec) and/or persisting ST elevation or depression >0.1 mV. The time from symptom onset to intensive care unit admission had to be
<48 hours. Patients were managed according to usual practice; treatment was not affected by participation in the registry. At the time of admission, when the first routine blood sample was drawn, an additional 50 ml were taken to create DNA and serum banks. The duration of recruitment was one month (31 days) per center for all patients and two months for diabetic patients, ranging from October 1 to December 24, 2005. Of the 374 centers in France that treated patients with AMI at that time, 223 (60 percent) participated in the registry. Among these, 100 centers recruited 1029 patients who contributed to measurement of both sPLA2 and
Lp-PLA2 activities. The characteristics of patients recruited in these 100 centers did not substantially differ from those recruited in the other centers. Written informed consent was provided by each patient. Follow-up was collected through contacts with the patients’ physicians, the patients or their family, and registry offices of their birthplace. A composite endpoint of death from any cause and nonfatal myocardial infarction was adjudicated by a committee whose members were unaware of patients’ medications, blood measurements and genotypes.
One-year follow-up was >99 percent complete.
The study was reviewed and received approval from the Committee for the Protection of
Human Subjects in Biomedical Research of Saint Antoine University Hospital.
Blood measurements
Lp-PLA2 activity
Lp-PLA2 activity was measured as previously described
4 . Briefly, 1 µl of plasma was diluted in 90 µl of HEPES-EDTA buffer (pH 7.4) and added with 10 µl of 3
[H]-acetyl-PAF-C16.
After 10 min-incubation at 37°C the reaction was stopped by trichloroacetic acid precipitation and free
3
[H]-acetate was measured in supernatants. Samples were measured in duplicate.
Pooled human EDTA plasma from 20 normolipidemic human subjects served as an internal
EURHEARTJ-D-11-02896R3 standard for all measurements. Lp-PLA2 activity is expressed in nmol/min per ml of plasma.
The within-assay variability was < 10%. sPLA2 activity
For sPLA2 activity, two methods were used. In PIVUS, plasma sPLA2 activity was measured on heparinated plasma samples using a selective fluorimetric method (AteroDX(tm) sPLA2
Activity, Aterovax, Paris, France). Results are expressed in Unit per mL of sample (U/mL), with one unit defined as the amount of sPLA2 enzyme which catalyses the release of one nmol of product in one min. Limit of detection of the assay is 17 U/mL with upper linear analytical range of 232 U/mL and functional sensitivity (20%) is 21 U/mL. Average withinrun variability and average intra-assay variability are 5.9% and 8.9%, respectively. In FAST-
MI, serum sPLA2 activity was measured using fluorescent substrate 1-hexadecanoyl-2-(1pyrenedecanoyl)-sn-glycero-3 phosphomethanol, sodium salt (Interchim, Montluçon, France), as previously described
5, 6
. One-hundred percent hydrolysis of the fluorescent substrate was measured using 0.1 U PLA2 from bee venom (Sigma Chemical Co). The hydrolysis of substrate in the absence of plasma was used as negative control and deduced from PLA2 activity. Plasma activity was expressed as nmol/min per mL. The minimum detectable activity was 0.10 nmol/min per mL. Intra- and inter-assay CV were less than 10%.
Evaluation of atherosclerosis in PIVUS study
Carotid artery ultrasound evaluation
The carotid artery was assessed by external B-mode ultrasound imaging (Acuson XP128 with a 10 MHz linear transducer, Acuson Mountain View, California, USA) among a subset of
PIVUS sample (n=954)
7
. The common carotid artery, the bifurcation and the internal carotid artery were visualized on both sides. A plaque was defined as a local thickening of the intimamedia thickness (IMT) being >50% thicker than the surrounding IMT. From this, it was recorded if plaque was present in none, one or both of the carotid arteries.
Magnetic resonance angiography
Imaging was performed on a 1.5 Tesla MRI system (Gyroscan Intera, Philips Medical
Systems, Best, the Netherlands) with a 25 mT/m gradient system, using the standard quadrature body coil among a subset of PIVUS sample (n=306)
7
. The whole body was scanned in the supine position using a 3D RF-spoiled T1-weighted gradient echo sequence before and after injection of 40 ml Gd-DTPA-BMA (OmniscanTM, GE Healthcare, Oslo,
EURHEARTJ-D-11-02896R3
Norway) at a rate of 0.6 ml/s. The acquired slice thickness was 4 mm with a resolution of 1.76 x 1.76 mm. Imaging did not include the coronary arteries.
The arterial tree was categorized into five territories: 1) the carotids including internal carotid artery (ICA) and common carotid artery, 2) the aorta including both the thoracic and abdominal part, 3) the renal arteries, 4) the pelvic/upper limbs including common iliac artery, external iliac artery (EIA), common femoral artery (CFA), superficial femoral artery (SFA) and popliteal artery (POP), 5) the lower legs including tibio-peroneal trunk (TPT), anterior tibial artery (ATA), peroneal artery (PA) and posterior tibial artery (PTA).
In order to obtain a comparable graded number reflecting the atherosclerosis in each territory, an atherosclerotic score was calculated for each territory. A normal vessel segment received null points, less than 50% stenosis was given one point and 50% reduction or more of the vessel diameter including occlusions was given two points. The points for the vessel segments in a territory were summarized. That sum was then divided with the maximum sum that would be achieved if all included segments had a more than 50% stenosis or occlusion.
A global total atherosclerosis score was defined as the sum of the five territories
8
. Aneurysms and vessel segments that could not be evaluated were excluded from the calculations.
Statistical analysis
The PIVUS sample
Non-normally distributed variables (such as sPLA2 activity, Lp-PLA2 activity, fasting glucose, serum triglycerides, CRP) were ln-transformed to achieve a normal distribution.
Differences between men and women regarding sPLA2 and Lp-PLA2 activities were evaluated by one-way ANOVA.
Relationships between sPLA2 and Lp-PLA2 activities and traditional risk factors were evaluated by linear regression, adjusting for gender. As a second step in this evaluation, we used a stepwise multiple fractional polynomial regression with p<0.05 for entry in the model to evaluate the independency of the relationships between the multiple risk factors waist circumference, serum triglycerides, BMI, CRP, HDL-C, LDL-C, triglycerides, GFR, fasting glucose, blood pressure, statin use, total energy and alcohol intake and exercise habits) and sPLA2 or Lp-PLA2 activity.
Ordinal logistic regression was used to evaluate the relationship between carotid atherosclerosis (three levels; none, one or two arteries) and sPLA2 or Lp-PLA2 activity.
EURHEARTJ-D-11-02896R3
Since about one-third of the participants did not show any stenosis at WBMRA, we used
Tobit regression to relate the degree of stenosis to sPLA2 or Lp-PLA2 activity. Tobit regression is used if the dependent variable is cencored at some value on the scale. In this case, cencoring was performed at the lower limit (zero) and a large number of individuals show this value justifying the use of Tobit regression.
Cox proportional hazard models were used to evaluate sPLA2 and Lp-PLA2 activities as predictors of all-cause mortality. The proportional hazard assumption was evaluated by
Schoenfeld´s test and by visual inspection of the presented graphs. We used age as the time scale, since all subjects were aged 70 years at study entry, and this will show the evolution of mortality in an intuitive way in figure 1. In the first set of models, adjustments were performed for gender only. In the next set of models, we adjusted for the other risk factors listed above. sPLA2 and Lp-PLA2 activities were evaluated both as continuous variables and following division in quartiles.
Goodness of fit was evaluated by the Hosmer-Lemeshow test with 10 groups. The additional value of adding sPLA2 activity to traditional risk factors was evaluated by C-statistics, likelihood ratio tests, and integrated discrimination improvement (IDI).
Two-tailed significance values were given with p< 0.05 regarded as significant. The statistical program package STATA 11 (STATA, College Station, CA; USA) was used.
The FAST-MI sample
Baseline demographic and clinical characteristics, treatment factors and therapeutic management during hospitalization were compared among the tertiles ranges of sPLA2 and
Lp-PLA2 activities with the use of a chi-square or Fisher’s exact tests for discrete variables, and by Wilcoxon sign-rank tests for continuous variables. We used multivariate Cox proportional-hazards model to assess the independent prognostic value of variables with the outcome events during the 1-year follow-up period. The multivariate model comprised sex, age, previous or current smoker, family history of coronary disease, previous history of hypertension, acute myocardial infarction, heart failure, renal failure, diabetes, blood pressure and heart rate at admission, Killip class, left ventricular ejection fraction, prior angioplasty or coronary bypass surgery, hospital management including reperfusion therapy, statins, betablockers, aspirin, clopidogrel, diuretics, digitalis glycosides, heparin, tertiles of sPLA2 or
Lp-PLA2 activity. The chosen variables are those significantly different in the univariate analysis or known cardiovascular risk or prognostic factors with P value <0.2 in a multiple
EURHEARTJ-D-11-02896R3 stepwise Cox proportional hazard model with forward selection. The proportional hazards assumption of the Cox model was verified (proportionality test P=0.48) using SAS program.
The assessment was performed by including the time-varying covariate, the interaction between each variable and the event time within the proc phreg. The proportional hazard assumption was also verified using Schoenfeld´s test (P=0.54) and by visual inspection of the presented graphs. Circulating levels of sPLA2 and Lp-PLA2 activities were log-transformed to remove positive skewness. Results are expressed as hazard ratios for Cox models with 95 percent confidence intervals. All statistical tests, performed using SAS software version 9.1 were two-sided.
References:
1. Hallstrom H, Melhus H, Glynn A, Lind L, Syvanen AC, Michaelsson K. Coffee consumption and CYP1A2 genotype in relation to bone mineral density of the proximal femur in elderly men and women: a cohort study. Nutr Metab (Lond) 2010;7:12.
2. Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective
Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb
Vasc Biol 2005;25:2368-75.
3. Cambou JP, Simon T, Mulak G, Bataille V, Danchin N. The French registry of Acute
ST elevation or non-ST-elevation Myocardial Infarction (FAST-MI): study design and baseline characteristics. Arch Mal Coeur Vaiss 2007;100:524-34.
4. Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease.
Circulation 2003;107:1579-85.
5. Mallat Z, Steg PG, Benessiano J, et al. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J Am Coll Cardiol
2005;46:1249-57.
6. Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T cell immunity in atherosclerosis.
Trends Cardiovasc Med 2007;In press.
7. Lind L, Andersson J, Hansen T, Johansson L, Ahlstrom H. Atherosclerosis measured by whole body magnetic resonance angiography and carotid artery ultrasound is related to arterial compliance, but not to endothelium-dependent vasodilation - the Prospective
EURHEARTJ-D-11-02896R3
Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Clin Physiol Funct
Imaging 2009;29:321-9.
8. Hansen T, Ahlstrom H, Wikstrom J, Lind L, Johansson L. A total atherosclerotic score for whole-body MRA and its relation to traditional cardiovascular risk factors. Eur Radiol
2008;18:1174-80.