File - Mr. Weiss` Science
advertisement

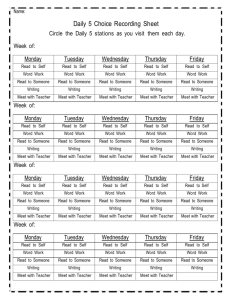
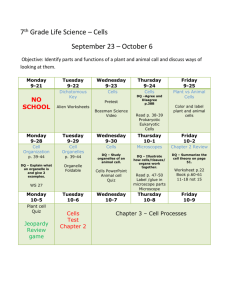
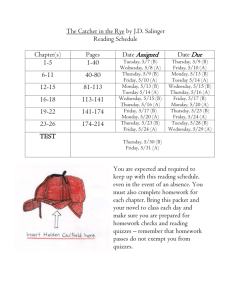
Unit 4: Balancing Chemical Equations and Stoichiometry 3. The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. c. Students know one mole equals 6.02x10 23 particles (atoms or molecules). d. Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic masses and how to convert the mass of a molecular substance to moles, number of particles, or volume of gas at standard temperature and pressure. e. Students know how to calculate the masses of reactants and products in a chemical reaction from the mass of one of the reactants or products and the relevant atomic masses. Date 1/5 Monday 1/6 Tuesday 1/7 Wednesday 1/8 Thursday 1/9 Friday 1/12 Monday 1/13 Tuesday 1/14 Wednesday 1/15 Thursday 1/16 Friday 1/20 Tuesday 1/21 Wednesday 1/22 Thursday 1/23 Friday 1/26 Monday Activity Ch 7.1/2 preview Lecture 7.1 + 7.2 Chemical Equations WS Ch 7.3 preview Lecture 7.3 Phet Lab: Balancing equations Balancing Equations 1 Review Packet Day 1 Review Packet Day 2 Review Packet Day 3 Review Packet Day 4 Balancing Equations 2 Team Learning Ch 7 CH 7 Rev Worksheet CA CH7 Review 1/27 Tuesday 1/28 Wednesday 1/29 Thursday 1/30 Friday 2/2 Monday 2/3 Tuesday 2/4 Wednesday 2/5 Thursday 2/6 Friday 2/10 Tuesday 2/11 Wednesday 2/12 Thursday 2/13 Friday 2/17 Tuesday 2/18 Wednesday 2/19 Thursday Chapter 7 Test Ch 9.1 preview Lecture 9.1 Mole to Mole WS TL 9.1 Ch 9.2 Preview Lecture 9.2 Stoichiometry WS 1 TL 9.2 Ch 9.3 preview Lecture 9.3 Phet Lab: stoichiometry Phet Lab: stoichiometry cont. Stoichiometry WS 2 Team Learning 9.3 CA CH9 Review 2/20 Friday 2/23 Monday Ch 9 rev worksheet Test Ch 9 Homework due -SRQ 7.1 #1 7.2# 1-4 -SRQ 7.3# 1-5 Review packet due! -CA CH7: #5, 7, 9, 11, 13, 33b/d, 34b, 35d - SRQ 9.1: 1, 2, 4, 5 - SRQ 9.2#1-6 -SRQ 9.3:# 3-5 -CA CH9 rev:# 8b/d, 9b/d, 11, 14f/g, 21, 26, 35b/d Unit 4: Balancing Chemical Equations and Stoichiometry 3. The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. c. Students know one mole equals 6.02x10 23 particles (atoms or molecules). d. Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic masses and how to convert the mass of a molecular substance to moles, number of particles, or volume of gas at standard temperature and pressure. e. Students know how to calculate the masses of reactants and products in a chemical reaction from the mass of one of the reactants or products and the relevant atomic masses. Date 1/5 Monday 1/6 Tuesday 1/7 Wednesday 1/8 Thursday 1/9 Friday 1/12 Monday 1/13 Tuesday 1/14 Wednesday 1/15 Thursday 1/16 Friday 1/20 Tuesday 1/21 Wednesday 1/22 Thursday 1/23 Friday 1/26 Monday Activity Ch 7.1/2 preview Lecture 7.1 + 7.2 Chemical Equations WS Ch 7.3 preview Lecture 7.3 Phet Lab: Balancing equations Balancing Equations 1 Review Packet Day 1 Review Packet Day 2 Review Packet Day 3 Review Packet Day 4 Balancing Equations 2 Team Learning Ch 7 CH 7 Rev Worksheet CA CH7 Review 1/27 Tuesday 1/28 Wednesday 1/29 Thursday 1/30 Friday 2/2 Monday 2/3 Tuesday 2/4 Wednesday 2/5 Thursday 2/6 Friday 2/10 Tuesday 2/11 Wednesday 2/12 Thursday 2/13 Friday 2/17 Tuesday 2/18 Wednesday 2/19 Thursday Chapter 7 Test Ch 9.1 preview Lecture 9.1 Mole to Mole WS TL 9.1 Ch 9.2 Preview Lecture 9.2 Stoichiometry WS 1 TL 9.2 Ch 9.3 preview Lecture 9.3 Phet Lab: stoichiometry Phet Lab: stoichiometry cont. Stoichiometry WS 2 Team Learning 9.3 CA CH9 Review 2/20 Friday 2/23 Monday Ch 9 rev worksheet Test Ch 9 Homework due -SRQ 7.1 #1 7.2# 1-4 -SRQ 7.3# 1-5 Review packet due! -CA CH7: #5, 7, 9, 11, 13, 33b/d, 34b, 35d - SRQ 9.1: 1, 2, 4, 5 - SRQ 9.2#1-6 -SRQ 9.3:# 3-5 -CA CH9 rev:# 8b/d, 9b/d, 11, 14f/g, 21, 26, 35b/d