CHEM120-83/84 - Wentworth Institute of Technology
advertisement

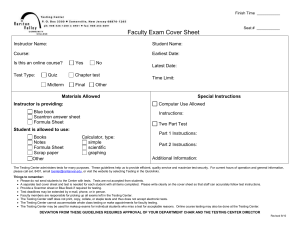
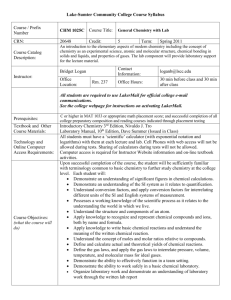
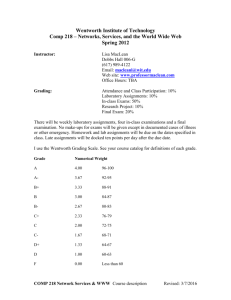
Fall 2010, Chem120, Chemistry for the Built Environment Wentworth Institute of Technology INSTRUCTOR: Donald G. Muldoon Adjunct Professor of Applied Math and Sciences Office location: Ira Allen Building 208 Email: muldoond@wit.edu OFFICE HOURS: Details on Blackboard Website (By appointment only) CREDIT HOURS: This is a four credit course, 3-2-4 MEETING TIMES: 9/7/2010-12/11/2010 Lecture: Chem 120-84 Tuesdays 5:00 – 6:50 p.m. Thursdays 7:00 - 7:50 p.m. Laboratory: Chem 120-85 Thursdays 5:00 – 6:50 p.m. Watson Hall 001 Wentworth Hall 210 Willis 203 COURSE DESCRIPTION: The Chem120 course serves as the introductory chemistry course for students in the Department of Civil, Construction, and Environment. The course provides a fundamental introduction to chemistry topics with a focus on the built environment. Fundamental principles of chemistry with emphasis on solving problems encountered in business and commerce. Topics include: the atomic model; writing, balancing; and predicting reactions; stoichiometry; the periodic table; properties of acids, bases, and salts; properties of aqueous solutions. Co-requisite: MATH 205 College Mathematics I. PREREQUISITE: Skills at Math I level REQUIRED TEXT BOOK: Introduction to Chemistry Richard C. Bauer, James P. Birk, and Pamela S. Marks, 2nd Edition McGraw Hill Higher Education Students must purchase either the electronic or the hard copy of the text book. Both items are available at the College Bookstore or on-line. 1 OTHER REQUIRED MATERIALS: A Laboratory Notebook is required for all assignments starting on week 2. Safety Goggles are required in all laboratory sessions starting on week 2 (safety glasses are not acceptable). Computer with internet access, word-processing and other basic software, blackboard/email accounts (use of computers during the lectures or laboratories will be at the instructor’s discretion). THE WENTWORTH BOOK STORE: 550 Huntington Ave Beatty Hall Boston, MA 02115 (617) 445-8814 Email: wit@bkstr.com; Shop online at: http://www.efollett.com/ INSTRUCTIONAL METHODOLOGIES: Lectures will be delivered using power point presentations, simulations, videos and movies. Student interaction and active participation is encouraged. Laboratory experiments will be related to fundamental chemistry principles and applications to the built environment. Students will be able to work in groups to design a final project for class presentation. COURSE LEARNING OUTCOMES: At the end of this course students will 1) Use fundamental chemical principles to make predictions about reactivity and general properties of materials in the built environment. 2) Apply fundamental chemical principles in laboratory experiments in a safe and professional manner 3) Understand the importance of record-keeping and have practiced its use during laboratories and/or lectures. 4) Be able to present experimental results accurately and concisely using technical narrative, graphs, and tables. 5) Be able to work independently and as part of a group to discuss and present fundamental chemistry concepts and applications to the built environment. 2 Chem 120 SCHEDULE: The following lecture and lab schedules may be changed at the instructor’s discretion based on students’ performance or for other extenuating circumstances. Laboratory handouts will be posted online. Use of safety goggles and laboratory notebooks will be required starting on week 2. Date Wk of 6th Wk of 13th Wk of 20th Wk of 27th Wk of 4th Wk of 11th Wk of 18th Sept Lecture Topic Laboratory Topics Matter, Energy and Scientific Inquiry, Chapter 1 Sept Atoms, Ions and The Periodic Table, Chapter 2 Sept Electron Structure of the Atom, Chapter 7 Sept Review and Exam 1 (150 points) Oct Chemical Bonding and Formulation, Chapters 3and 8 Oct Chemical Composition, Chapter 4 Oct Wk of Oct 25th Wk of Nov 1st Wk of Nov 8th Wk of Nov 15th Wk of Nov22nd Wk of Nov29th Wk of Dec 6th Wk of Dec 13th Chemical Reactions and Equations, Chapter 5 Review and Exam 2 (150 points) Check in and Required Sign-up of Safety Agreement by Students Safety Lab Overview Measurement and Density (Penny Lab) Reactivity Series of Metals Alloy lab (The Gold Penny Lab) Ionic and Covalent Properties lab Separation of Mixture components by Paper Chromatography Solutions/Dilutions and Spectrophotometer lab (Beer’s Law) Quantities in Chemical Reactions, Magnesium Oxide Synthesis Chapter 6 Acids and Bases, Chapter 13 No Laboratory (Veteran’s Day) Oxidation-Reduction and Corrosion, Chapter 14 Review and Exam 3 (150 points) Acid/Base Titration in Cement Students’ Final Project (150 points) Galvanic Cell versus Electrolysis Review For The Final Exam Silver Mirror and Plating; Check Out No Laboratory (Thanksgiving) Final Exam (200 points, date TBA) 3 GRADING POLICY: Lectures: Three lecture exams* ................................ ……. 45% (150 each exam) *Final Project ................................................... 15% (150 points) *Grade of the final project substitutes for the lowest lecture exam grade Final Exam (cumulative) .................................. .. 20% (200 points) Weekly Homework/In-class Work 10% (100 points) Instructor’s discretion (Attendance, Participation) 10% (100 points) Labs: Lab Attendance/ Notebook ...........................…. 15% (150 points) 100% (1000 points total) PLEASE NOTE THAT TO PASS THE COURSE, THE AVERAGE OF THE LECTURE EXAMS/FINAL PROJECT/FINAL EXAM HAS TO BE A PASSING GRADE (60% or higher) Guidelines, Handouts and Rubric for Final project and lab notebook will be provided by your instructor. Grades for the course will be reported as: 96-100% (A); 92-95% (A-); 8891% (B+); 87-84%(B); 80-83% (B-); 76-79% (C+); 72-75% (C); 68-71% (C-); 64-67% (D+); 60-63% (D); Below 60% (F) ATTENDANCE POLICY: Attendance will be monitored as described in the catalog http://www.wit.edu/prospective/catalog/2010/Attendance.html) (see ‘The instructor will use his or her discretion for all extenuating circumstances regarding attendance. It is the responsibility of the student to notify an instructor of all absences (anticipated or not) and provide documentation regarding illness or absence’. DROP/ADD: The drop/add period for day students ends on Friday of the first week of classes. Dropping and/or adding courses is done online. Courses dropped in this period are removed from the student’s record. Courses to be added that require written permission, e.g. closed courses, must be done using a Drop/Add form that is available in the Student Service Center. Nonattendance does not constitute dropping a course. If a student has registered for a course and subsequently withdraws or receives a failing grade in its prerequisite, then the student must drop that course. In some cases, the student will be dropped from that course by the Registrar. However, it is the student’s responsibility to make sure that he or she meets the course prerequisites and to drop a course if the student has not successfully completed the prerequisite. The student must see his or her academic advisor or academic department head for schedule revision and to discuss the impact of the failed or withdrawn course on the student’s degree status 4 MAKE-UP POLICY: If a student misses an examination due to reasonable and verifiable circumstances, she/he will be able to make-up the exam missed during the instructor’s office hours. There is no make-up for missing laboratory assignments. ACADEMIC SUPPORT: The Center for Teaching and Learning (CTL) assists all Wentworth students with academic challenges in the areas of mathematics, science, technical courses specific to majors, and writing. The CTL is a supportive and safe learning environment for students looking to improve or maintain their academic standing. In this student-based learning environment, students can receive individual help with their studies, meet and work in study groups, or go on-line to find resources to assist them in meeting their goals for academic success. It includes tutors in many subjects, online writing assistance and workshops. Make appointments at www.wit.edu/academics/resources or through L-Connect. ACADEMIC HONESTY STATEMENT: “Students at Wentworth are expected to be honest and forthright in their academic endeavors. Academic dishonesty includes cheating, inventing false information or citations, plagiarism, tampering with computers, destroying other people’s studio property, or academic misconduct” (Academic Catalog). See your catalogue for a full explanation. STUDENT ACCOUNTABILITY STATEMENT: Repeated cheating and/or plagiarism will be reported to the Institute and will result in a grade of F for the class. In any event, copying answers from the exams of other students is not advisable, since the order of the questions and the possible answers will be different among different exams. When writing original works or projects, citations should be included to identify the sources of information. If you have questions about what cheating and plagiarism means in a science course, just ask your instructor. DISABILITY SERVICES STATEMENT: Any student who thinks s/he may require a disability-related accommodation for this course should contact me privately to discuss his/her specific needs. Disability Services coordinates reasonable accommodations for students with documented disabilities. They are located in Watson Hall 003 (the Counseling Center) and can be contacted at 617-989-4390 or counseling@wit.edu. For more information on acceptable documentation and the Disability Services process, visit the Disability Services website at www.wit.edu/disabilityservices. 5