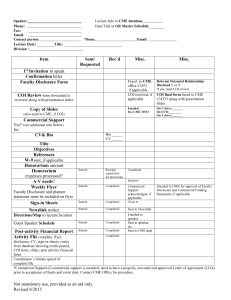
Faculty Disclosure Form
advertisement

Faculty Disclosure Form – Page 1 Name: Activity Title: Date of Activity Part 1: To be completed by the course director, committee member, speaker, etc. “Relevant financial relationships” are those in which an individual (including spouse/domestic partner) has both: 1. A personal financial relationship (any amount) with a commercial interest producing health care goods/services in the past 12 months (whether relationship has now ended or is currently active.) 2. Control in planning or presenting educational content addressing specific products/agents/devices of the commercial interest (not simply a whole class of products or content about the whole disease class) Disclosure: Regarding your role in the CME activity listed above, have you (or your spouse/partner) had a relevant personal financial relationship in the last 12 months with the manufacturer of the products or services that will be discussed in the CME activity or in your presentation? No Skip to OFF-LABEL DISCUSSION and DECLARATION sections below Yes Please list your disclosures: Nature of Relevant Financial Relationship (include all those that apply) Commercial Interest Example: Company X Type of Personal Financial Relationship. Stating what was your role and what was received? Role: Speaker. Received: Honoraria Role: Advisory Board Member: Received: Consultant Fee Relationship Status Ended or Current? Current Additional information may be requested to resolve any conflict of interest. Disclosure will be made to the participants prior to the activity. Please describe any additional information relevant to the disclosure below. How is your personal financial relationship related to the educational content that you are presenting? OFF-LABEL DISCUSSION: I will be discussing a product which is still investigational or not labeled for the use under discussion. Please explain: DECLARATION: 1. All the recommendations involving clinical medicine in a CME activity are based on evidence that is accepted within the profession of medicine as adequate justification for their indications and contraindication in the care of patients. 2. All scientific research referred to, reported or used in CME in support or justification of a patient care recommendation must conform to the generally accepted standards of experimental design, data collection and analysis. 3. I will uphold academic standards to insure balance, independence, objectivity and scientific rigor. 4. I agree to comply with the requirements to protect health information under the Health Insurance Portability and Accountability Act of 1996 (HIPPAA). 5. I agree to obtain the necessary copyright permission(s) if any portion of my CME activity materials that I prepare is not my original work or for which I do not hold the copyright. 6. I understand that my CME presentation may be peer-reviewed for fair balance and validation of content and will be evaluated by participants for fair balance. Signature:__________________________________________________ Date: _________________________ Part 2: Must be completed by the Course Director if speaker indicated a current financial relationship or the disclosure information is verbally communicated to the audience Conflict of Interest Circumstances create a conflict of interest are when an individual has an opportunity to affect CME content about products or services of a commercial interest with which he/she has a financial relationship. The act of disclosing to the participants, in itself, does not resolve the conflict of interest. Please see the attached page for approved ways to resolve conflict of interests. Please indicate how you will resolve this conflict of interest and explain why this is an appropriate method. As the course director, I propose the following to resolve the conflict of interest: As the course director, I attest that the speaker’s disclosure information was communicated on the promotional flyer or verbally during the speaker’s introduction. Signature:__________________________________________________ Date: _________________________ Faculty Disclosure Form – Page 2 Resolving Conflict of Interest Conflicts of interest may be resolved by: 1. Altering financial relationships – Individuals may change their relationships with commercial interests (e.g., discontinue contracted services). Thereby eliminating any bias into the CME content. Updated 11/22/11 2. Choosing someone else to control that part of the content. If a proposed presenter or planner has a conflict of interest related to the content, someone else who does not have a relationship to the commercial interests related to the content may present or plan this part of the content. 3. Change the focus of the CME activity so that the content is not about products or services of the commercial interest that is the basis of the conflict of interest. 4. Change the content of the person’s assignment so that it is no longer about products or services of the commercial interest. For example, an individual with a conflict of interest regarding products for treatment of a condition could address the pathophysiology or diagnosis of the condition, rather than therapeutics. 5. Limit the content to a report without recommendations. If an individual has been funded by a commercial company to perform research, the individual’s presentation may be limited to the data and results of the research. Someone else can be assigned to address broader implications and recommendations. 6. Limit the sources for recommendations. Rather than having a person with a conflict of interest present personal recommendations or personally select the evidence to be presented, limit the role of the person to reporting recommendations based on formal structured reviews of the literature with the inclusion and exclusion criteria (‘evidence-based’). 7. Independent Content Validation – Conflict of interest may be resolved if the CME material is peer reviewed and: All recommendations involving clinical medicine are based on evidence that is accepted within the profession of medicine as adequate justification for indications and contraindications in the care of patients. All scientific research referred to, reported or used in the CME activity in support or justification of patient care recommendations conforms to the generally accepted standards of experimental design, data collection and analysis.