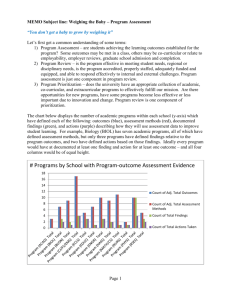
NCQA 2014 Standards PPs
advertisement

NCQA 2014 Standards Documented Process Submission - Policies and Procedures Based on April 2015 Update June Levine MSN, BSN RN Not all factors associated with specific elements require submission of Documented Processes. A Documented Process is defined as: Written procedures, protocols, processes, & workflow forms (not explanations). The same factors and/or other factors will require the submission of other documents. However, in submitting the NCQA required reports, records, files and/or information materials the practice may want to have policies and procedures to help assure practice continuity and consistency. The names of the policies and procedures identified below are titles that would be applicable to the intent of the Factor. However, you may have policy and procedures with a different name that includes or can include the required information. They should be reviewed against the standard explanation and required documentation for each applicable element. NCQA has these requirements when submitting policies, procedures and processes. “Policies, procedures and processes must be in place for at least 3 months prior to survey submission Intended for practice staff to ensure a systematic approach to patient care and practice operations If the documented process has been updated, the practice must provide: New and previous documented process or where changes have occurred Date of update” See more at: http://www.ncqa.org/Programs/Recognition/Practices/PatientCenteredMedicalHomePCMH/DuringEarnItPCMH/OtherPCMHResources/DocumentationReportingPe riodsPCMH2014FAQs.aspx#sthash.70Znny7P.dpuf Documented Process Submission - Policies and Procedures June 2015 Page 1 of 4 Standard 1. Patient-Centered Access Element A. Patient Centered Appointment Access B. 24/7 Access to Clinical Advice C. Electronic Access Standard 2. Team Based Care Element A. Continuity B. Medical Home Responsibilities C. Culturally and Linguistically Appropriate Services D. The Practice Team Documented Process Submission - Policies and Procedures Policy & Procedure for a Documented Process Factors 1-6. PP: Appointment Scheduling One PP could document requirements for all 6 factors. Labeling each section would distinguish requested information & provide clarity for submission. Factors 1-4. PP: Access to Clinical Advice One PP could document requirements for all 4 factors. Labeling each section would distinguish requested information & provide clarity for submission. Factors 1-6. No documented process required Policy & Procedure for a Documented Process Factor 1. PP: Selection of a Personal Provider Factor 3. PP: Orienting Patients to the Practice Factor 4. PP: Preventive, Acute and Chronic Care Management for the Adolescent & Young Adult Factors 1-8. PP: Orienting Patients to the Practice The same PP (under 2A) above can address this Element. A policy and procedure that addresses Factor 3: PP: Interpretive Services Factors 1, 5, 6, 7. PP: Clinical Staff Roles or dated descriptions of staff positions Factor 3 & 8. PP: Staff Meetings or Huddles. You could include Huddles in a PP titled Staff Meetings if Huddles are limited to only certain providers &/or are limited in their scope. Factor 9. PP: Quality Improvement Factor 10. PP: Patient/Family Involvement in Practice Improvement June 2015 Page 2 of 4 Standard 3. Population Management Element A. Patient Information B. Clinical Data C. Comprehensive Health Assessment D. Use Data for Population Management E. Implement Evidence-Based Decision Support Standard 4. Care Management & Support Standard 5. Care Coordination & Care Transitions Element A. Identify Patients for Care Management B. Care Planning & Self-Care Support C. Medication Management D. Use Electronic Prescribing E. Support Self-Care & Shared Decision Making Policy & Procedure for a Documented Process Factor 14. PP: Capturing Data for Population Management (the data asked for is outlined in factors 1-13) No specific PPs required No specific PPs required No specific PPs required No specific PPs required Policy & Procedure for a Documented Process Factors 1-5. PP: Identifying Patients for Care Management No specific PPs required No specific PPs required No specific PPs required No specific PPs required Element A. Test tracking and Follow-Up Policy & Procedure for a Documented Process Factor 1-6 PP: Test Tracking and Follow-up B. Referral Tracking & Follow-Up C. Coordinate Care Transitions Factors 5,6,8,10. PP: Referral Tracking and Follow-up Factor 1. PP: Identifying patients who have been hospitalized or have had an ER visit. Factor 2 PP: Patient Clinical Information Provided to Hospitals and ERs. Factor 3. PP: Obtaining hospital discharge summaries. Documented Process Submission - Policies and Procedures June 2015 Page 3 of 4 5. Care Coordination & Care Transitions, continued C. Coordinate Care Transitions, continued Factor 4. PP: Patient follow-up after a hospital admission or ER visit Factor 5. PP: Two-way Communication with Hospitals. Factor 6. PP: Release of Patient Information. Standard 6 Element 6. Performance Measurement A. Measure Clinical Quality & Quality Improvement Performance B. Measure Resource Use & Care Coordination C. Measure Patient/Family Experience D. Implement Continuous QI Improvement E. Demonstrate Continuous Quality Improvement F. Implement Continuous QI Improvement G. Use Certified EHR Technology Factors: Document process for: Factors 1 – 4. No documented processes required Documented Process Submission - Policies and Procedures June 2015 Factor 1 & 2. No documented processes required Factors 1-4. No documented processes required Factors 1-7. No documented processes required Factors 1 – 4. No documented processes required Factors 1-4. No documented processes required Factors 1-10. No documented processes required Page 4 of 4