Concomitant Thionation and Reduction of Graphene
advertisement

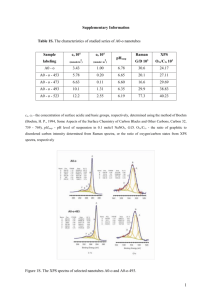
S1 Concomitant Thionation and Reduction of Graphene Oxide Through Solid/Gas Metathetical Sulfidation Reactions at High Temperatures Ki-Wan Jeon and Dong-Kyun Seo* Department of Chemistry and Biochemistry, Arizona State University Tempe, AZ 85287-1604, USA Email: dseo@asu.edu Supplemental Materials Figure S 1. Raman spectra of (a) graphite flake, (b) GO, (c) ttGO, and (d) RGOS. The G bands appear around 1600 cm-1, while the D bands are visible around 1340 cm-1 for all the samples except the graphite flake. Graphite flake exhibits a 2D band at 2712 cm-1.1 The positions of the S2 G band for GO, ttGO, and RGOS are higher in frequency than that for graphite flake, which has been attributed to significant defects on graphene sheet.1a, 2 The Full Widths at Half Maximum (FWHMs) of the G band of GO, ttGO, and RGOS are much larger than that of graphite flake, which is due to a higher degree of atomic disorder.1b, 3 The ID/IG ratio of RGOS (1.11) is almost the same as that of GO (1.08). S3 Figure S 2. XPS survey scans of air-dried GO (left) and RGOS (right). See the main text for the details. Figure S 3. Representative SEM image and EDS spectrum of air-dried RGOS. See the main text for the details. S4 Figure S 4. ATR FT-IR spectra for ethanol (black line) and GO solution dispersed in ethanol (blue line) and RGOS dispersed in ethanol (red line). The RGOS dispersion shows two weak peaks at 617 and 717 cm-1 which are attributed by C–S stretching vibration.4 The C–S stretching vibration is known to have a low intensity.5 S5 Figure S 5. UV-Vis spectra of L-cysteine standard solutions with various concentrations and a mixture of RGOS and DTNB in aqueous Tris-HCl buffer at pH 8 in the Ellman assay experiment.6 Figure S 6. Plot of optical absorbance at 413 nm versus thiol concentration obtained from Figure S 5. Black dots and white dot represent the standard solutions and a sample solution, respectively. The straight line (red) is a linear least square fit of the experimental data (Rp = 0.998). S6 Figure S 7. XPS survey scan (top-left) and high-resolution XPS spectra of core level region of C1s (top-right), O1s (bottom-left), and S2p (bottom-right) of RGOS after heat treatment at 600 °C. S7 Figure S 8. UV-Vis absorption spectra of GO (black) and RGOS dispersions (red). The red-shit of the peak maximum is consistent with the reduced nature of the graphene sheets.7, 8 S8 References (1) (a) Nemanich, R. J.; Solin, S. A., Phys. Rev. B 1979, 20, 392-401. (b) Knight, D. S.; White, W. B., J. Mater. Res. 1989, 4, 385-393. (c) Yan, J.-A.; Ruan, W. Y.; Chou, M. Y., Phys. Rev. B 2008, 77, 125401. (2) (a) Tuinstra, F.; Koenig, J. L., J. Chem. Phys. 1970, 53, 1126-1130. (b) Livneh, T.; Haslett, T. L.; Moskovits, M., Phys. Rev. B 2002, 66, 195110. (c) Cançado, L. G.; Pimenta, M. A.; Neves, B. R. A.; Dantas, M. S. S.; Jorio, A., Phys. Rev. Lett. 2004, 93, 247401. (3) Kudin, K. N.; Ozbas, B.; Schniepp, H. C.; Prud'Homme, R. K.; Aksay, I. A.; Car, R., Nano Lett. 2008, 8, 36-41. (4) (a) Silverstein, R.; Morril, T. Spectrophotometric identification of organic compounds; Wiley and Sons New York: 1974. (b) Bellamy, L. J. The infra-red spectra of complex molecules; London : Chapman and Hall ; New York : Wiley: 1975. (5) Chin, H. C., Carbon 1981, 19, 175-186. (6) Ellman, G. L., Arch. Physiol. Biochem. 1959, 82, 70-77. (7) Mathkar, A.; Tozier, D.; Cox, P.; Ong, P.; Galande, C.; Balakrishnan, K.; Leela Mohana Reddy, A.; Ajayan, P. M., J. Phys. Chem. Lett. 2012, 3, 986-991. (8) Shin, H.-J.; Kim, K. K.; Benayad, A.; Yoon, S.-M.; Park, H. K.; Jung, I.-S.; Jin, M. H.; Jeong, H.-K.; Kim, J. M.; Choi, J.-Y.; Lee, Y. H., Adv. Funct. Mater. 2009, 19, 19871992.