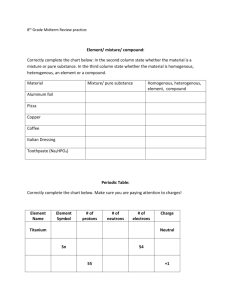
ATOMS into IONS Predict the charge on the most common
advertisement

ATOMS into IONS 1. Predict the charge on the most common monatomic ion formed by each element. a. Sodium b. Selenium c. Barium d. Rubidium e. Nitrogen f. Aluminum ATOMS into IONS 1. Predict the charge on the most common monatomic ion formed by each element. a. Sodium b. Selenium c. Barium d. Rubidium e. Nitrogen f. Aluminum 2. What is the total number of electrons present in each ion? a. F− b. Rb+ c. Ce3+ d. Zr4+ e. Zn2+ f. Kr2+ g. B3+ 2. What is the total number of electrons present in each ion? a. F− b. Rb+ c. Ce3+ d. Zr4+ e. Zn2+ f. Kr2+ g. B3+ 3. Predict the charge on the most common monatomic ion formed by each element. a. Chlorine b. Phosphorus c. Scandium d. Magnesium e. Arsenic f. Oxygen 3. Predict the charge on the most common monatomic ion formed by each element. a. Chlorine b. Phosphorus c. Scandium d. Magnesium e. Arsenic f. Oxygen 4. What is the total number of electrons present in each ion? a. Ca2+ b. Se2− c. In3+ d. Sr2+ e. As3+ f. N3− 4. What is the total number of electrons present in each ion? a. Ca2+ b. Se2− c. In3+ d. Sr2+ e. As3+ f. N3− IONIC COMPOUNDS IONIC COMPOUNDS 1. Gallium becomes a cation with a 3+ charge. A. What is the formula when it forms an ionic compound with Arsenic? 1. Gallium becomes a cation with a 3+ charge. A. What is the formula when it forms an ionic compound with Arsenic? 2. Europium becomes a cation with a 3+ charge. A. What is the formula when it forms an ionic compound with oxygen? 2. Europium becomes a cation with a 3+ charge. A. What is the formula when it forms an ionic compound with oxygen? 3. Calcium combines with chlorine to form an ionic compound A. What is the charge for each of the ion? B. What is the formula for the ionic compound? 3. Calcium combines with chlorine to form an ionic compound A. What is the charge for each of the ion? B. What is the formula for the ionic compound? 4. Lithium and nitrogen combine to form an ionic compound A. What is the charge for Li and N? B. What is the formula for the ionic compound? 4. Lithium and nitrogen combine to form an ionic compound A. What is the charge for Li and N? B. What is the formula for the ionic compound? 5. Aluminum and oxygen combine to form an ionic compound A. What is the charge for Al and O? B. What is the formula for this ionic compound? 5. Aluminum and oxygen combine to form an ionic compound A. What is the charge for Al and O? B. What is the formula for this ionic compound? 6. Potassium and cyanide combine to form an ionic compound A. What are the charges for each? B. What is the formula for this ionic compound? 6. Potassium and cyanide combine to form an ionic compound A. What are the charges for each? B. What is the formula for this ionic compound? 7. Calcium and hypochlorite combine to form an ionic compound A. What are the charges for each? B. What is the formula for the compound? 7. Calcium and hypochlorite combine to form an ionic compound A. What are the charges for each? B. What is the formula for the compound? 8. Ammonium and sulfate combine to form an ionic compound A. What are the charges for each? B. What is the formula for the compound? 8. Ammonium and sulfate combine to form an ionic compound A. What are the charges for each? B. What is the formula for the compound? NAMING IONIC COMPOUNDS NAMING IONIC COMPOUNDS 1. Write the names of the following compounds A. Ca3N2 B. FeI3 C. Na2O D. AlCl3 E. SrO 1. Write the names of the following compounds a. Ca3N2 b. FeI3 c. Na2O d. AlCl3 e. SrO 2. Write the formulas for the following ionic compounds A. Copper(II)oxide B. Sodium fluoride C. Zinc chloride D. Aluminum sulfide E. Potassium nitride 2. Write the formulas for the following ionic compounds a. Copper(II)oxide b. Sodium fluoride c. Zinc chloride d. Aluminum sulfide e. Potassium nitride 3. Write the names of the following compounds a. Mg(HCO3)2 b. Sr(HSO4)2 c. (NH4)2SO4 d. Ca(NO3)2 e. CuSO4 3. Write the names of the following compounds a. Mg(HCO3)2 b. Sr(HSO4)2 c. (NH4)2SO4 d. Ca(NO3)2 e. CuSO4 4. Write the formulas for the following ionic compounds a. Aluminum Nitrate b. Magnesium Phosphate c. Ammonium acetate d. Potassium Permanganate 4. Write the formulas for the following ionic compounds a. Aluminum Nitrate b. Magnesium Phosphate c. Ammonium acetate d. Potassium Permanganate 5. Write the names of the following compounds a. Ca(NO3)2 b. CuSO4 c. CuNO3 d. Na2CO3 e. K2Cr2O7 f. Mg(NO3)2 g. Rb3PO4 5. Write the names of the following compounds a. Ca(NO3)2 b. CuSO4 c. CuNO3 d. Na2CO3 e. K2Cr2O7 f. Mg(NO3)2 g. Rb3PO4 6. Write the formula for each compound. a. barium chloride b. sodium carbonate c. iron(III) hydroxide 6. Write the formula for each compound. a. barium chloride b. sodium carbonate c. iron(III) hydroxide 7. Turn each element into an ion. 8. Then combine them to make a compound. 9. Then name the compound a. calcium and chlorine b. lithium and oxygen c. potassium cation and iodine d. calcium and hypochlorite e. sodium cation and bicarbonate f. ammonium cation and sulfate 7. Turn each element into an ion. 8. Then combine them to make a compound. 9. Then name the compound a. calcium and chlorine b. lithium and oxygen c. potassium cation and iodine d. calcium and hypochlorite e. sodium cation and bicarbonate f. ammonium cation and sulfate