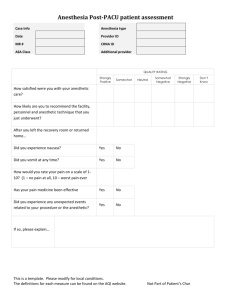
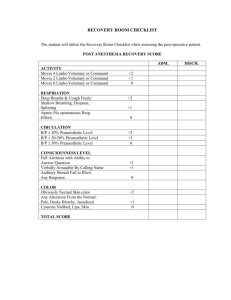
AMERICAN SOCIETY OF ANESTHESIOLOGISTS Anesthesiology
advertisement

AMERICAN SOCIETY OF ANESTHESIOLOGISTS Anesthesiology Continuing Education Program Answer Key Issue 4A 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. B C B C C D D D B D A B B A A C B C A C C C D D A C D D B B C D C A 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. C B B B C D D A B D D C D D A C A D D C D A D D D A C B C D C D B 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. D B B B D C D C D D A D B D A B B A B C D A C C B D D C A B B D D ITEM 1 A 72-year-old man is transferred to the intensive care unit after surgery to repair an abdominal aortic aneurysm. He remains intubated and mechanically ventilated, but his muscle relaxant has been reversed and he is starting to breathe. Tracings of the flow, volume, and airway pressure waveforms from the mechanical ventilator are shown. The tracings show the ventilator set in the assist-control (AC) mode with volume preset. In Figure 1, the negative deflection evident in the airway pressure trace for the second breath (labeled “B” in the tracing) represents a spontaneous inspiratory effort. The absence of any negative deflection prior to the onset of flow in the first breath (labeled “A” in the tracing) indicates that this breath is initiated purely by the ventilator. Figure 1. Tracing shows the ventilator set in the AC mode with volume preset. A represents a machineinitiated breath and B represents a spontaneous breath. AC ventilation may be either volume preset or pressure preset. In volume preset ventilation, identical tidal volumes are delivered with every breath but peak inspiratory pressure may vary with changes in pulmonary compliance or airway resistance. In pressure preset ventilation, volume is delivered until the preset pressure is attained; changes in pulmonary compliance or airway resistance may produce changes in delivered tidal volume. In AC ventilation, the mandatory ventilator rate is set by the clinician; spontaneous inspiratory efforts may result in a total respiratory rate greater than the preset rate. In 1 volume-preset AC ventilation, every breath, whether initiated in response to a spontaneous inspiratory effort (“assisted breath”) or purely machine-initiated (“control breath”), results in the same tidal volume. In pressure support ventilation the patient determines the respiratory rate, inspiratory time, peak flow, and tidal volume. Spontaneous breaths are augmented by a positive inspiratory pressure above the baseline. Pressure support ventilation is commonly used in conjunction with continuous positive airway pressure (CPAP), in which case the inspiratory pressure is added to the CPAP. Pressure support ventilation relies on an intact respiratory drive; without a specified ventilator rate, a patient may be apneic despite being on a ventilator. Patient-ventilator interaction may be improved by the patient’s ability to set his or her own flow. Bilevel ventilation is characterized by the setting of two levels of positive end-expiratory pressure (PEEP). The patient is able to spontaneously breathe at any point in the ventilatory cycle. Airway pressure release ventilation is a form of bilevel ventilation where the expiratory phase (in which the ventilator cycles from the higher to the lower level of PEEP) is very short and the vast majority of spontaneous breathing occurs at the higher PEEP level. Examples of mechanical ventilation modes are shown in Figure 2. 2 Figure 2. Examples of different modes of mechanical ventilation. 3 REFERENCES 1. Tobin MJ. Advances in mechanical ventilation. N Engl J Med. 2000; 344:1986-1996. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:28202825. 3. Murray MJ, Coursin DB, Pearl RG, et al. Critical Care Medicine: Perioperative Management. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002; 447-459. 4. McIntyre NR, Branson RD. Mechanical Ventilation. Philadelphia: WB Saunders; 2001:51-84. 5. Critical Care Medicine Mechanical Ventilation Tutorials. Available at: www.ccmtutorials.com/rs/mv/index.htm. Accessed February 2007. ITEM 2 Etomidate is a carboxylated imidazole-containing compound chemically unrelated to any other drug used for the intravenous induction of general anesthesia. Only the dexto-isomer is pharmacologically active. The mechanism of action is probably enhancement of GABA-induced central nervous system inhibition. Etomidate is formulated in a 0.2% solution with 35% propylene glycol. The propylene glycol solution is acidic and causes pain on injection. Awakening after administration of etomidate is due to redistribution from the brain to inactive tissue sites. The drug is rapidly metabolized to an inactive compound by hepatic microsomal enzymes and plasma esterases, and the metabolite is excreted in the urine and bile. Etomidate may preserve hemodynamic stability better than propofol or sodium thiopental. Side effects include myoclonus, nausea and vomiting, and adrenal suppression. Etomidate causes adrenal suppression by producing a dose-dependent inhibition of the conversion of cholesterol to cortisol (Figure 1). The inhibited enzyme is 11 beta-hydroxylase, which transforms 11-deoxycortisol into cortisol. Infusions of etomidate used for sedation have been associated with deaths due to adrenal suppression, and use of the drug as an infusion is not recommended. The use of etomidate as an induction agent in critically ill patients is controversial because of the relative adrenal insufficiency it causes. 4 Figure 1. Biosynthesis of cortisol and aldosterone. Etomidate affects cortisol-aldosterone synthesis by its action on 11 beta-hydroxylase and, to a lesser extent, 17 alpha-hydroxylase. Use with permission, from Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:Figure 10-24. REFERENCES 1. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:145-148. 2. Jackson WL Jr. Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: A critical appraisal. Chest. 2005; 127:1031-1038. 3. Mohammad Z, Afessa B, Finkielman JD. The incidence of relative adrenal insufficiency in patients with septic shock after the administration of etomidate. Crit Care. 2006; 10(4):R105. ITEM 3 Epidermolysis bullosa (EB) is a vesicobullous disorder resulting in separation of skin layers and bullae formation (Figure 1). Bullae formation can occur with minimal amounts of skin trauma even from just friction or pressure to the skin. EB is a genetic disease with variable penetrance. Patients with EB are at increased risk for future development of squamous cell carcinoma. 5 Clinical features of patients with EB (see Figure 1) include: decreased mouth opening due to scarring of the oral cavity pharyngeal bullae causing upper airway obstruction sublottic scarring resulting in lower airway obstruction corneal ulceration or perforation esophageal strictures gastroesophageal reflux disease malnutrition anemia of chronic disease scarring of extremities dysplastic teeth atrophic hair and nails secondary skin infections Figure 1. Epidermolysis bullosa. Image courtesy of Advanced Dermatology Education Server, a project of the Graduate Institute of Medical Informatics in Taipei Medical University, Taipei, Taiwan. Available online at http://ades.tmu.edu.tw/english/sp-topic/jeb/jeb-1.jpg. Key anesthetic management principles include minimizing friction and pressure to all skin surfaces. This would include procedures involving vascular line placement, regional anesthesia and airway manipulation. Many events in the perioperative period can produce bullae formation in patients with EB. Products with tape or adhesives may cause friction significant enough to produce bullae formation. Specific examples include electrocardiographic (ECG) leads, pulse oximeter probes, and tape used for intravenous lines, 6 tracheal tubes, and eyes. Any event that produces skin pressure or friction should be avoided in patients with EB. Suggested anesthetic management for the patient with EB includes: Prepare for possible difficult airway Evaluate for increased risk of aspiration Avoid all tape or adhesives Cut off adhesives to ECG leads and pulse oximetry probes Prepare for possible difficult intravenous access Secure IV lines and tracheal tubes with gauze or suture Use ointment for eye lubrication Pad extremities before placing blood pressure cuffs or tourniquets Lubricate face mask prior to placement, avoid friction during use Lubricate tracheal tube and laryngoscope Avoid wiping skin with skin prep solution, use blotting motion Generously pad dependent portions and extremities It has been well reported that patients with EB can tolerate tracheal intubation without significant morbidity or bullae formation when appropriate precautions are taken. Patients with EB are not at increased susceptibility for malignant hyperthermia or serum potassium disorders. REFERENCES 1. Iohom G, Lyons B. Anaesthesia for children with epidermolysis bullosa: A review of 20 years’ experience. Eur J Anaesthesiol. 2001; 18:745-754. 2. Behrman RE, Kliegman R, Jenson HB. Nelson Textbook of Pediatrics. 17th ed. Philadelphia: WB Saunders. 2004:2184-2186. 3. Baum VC, O’Flaherty JE. Anesthesia for Genetic, Metabolic, and Dysmorphic Syndromes of Childhood. Philadelphia: Lippincott Williams and Wilkins; 1999:99-100. ITEM 4 Clopidogrel (Plavix) irreversibly inhibits platelet aggregation by binding to the ADP receptor located on the platelet cell membrane. It also inhibits the binding of fibrinogen to the glycoprotein IIb/IIa receptor. Its elimination half-life is 7.7 hours after a single 75 mg oral dose. Ticlopidine, not clopidogrel, is associated with pancytopenia. Thrombotic thrombocytopenic purpura, one of the more common severe complications of clopidogrel therapy, generally occurs within the first two weeks of initiating therapy. A single loading dose of clopidogrel (375 mg) has clinically significant effects on platelet aggregation. The clinical effects of clopidogrel require sophisticated testing that is not routinely available outside of large medical centers. Platelet inhibition due to clopidogrel persists for several days after cessation of therapy. The American Society of Regional Anesthesia and Pain Medicine recommends that clopidogrel be stopped seven days before performing a neuraxial block. 7 REFERENCES 1. Broadman LM. Anticoagulation and regional anesthesia. Refresher Courses in Anesthesiology. American Society of Anesthesiologists. 2005; 33:31-47. 2. Coukell AJ, Markham A. Clopidogrel. Drugs. 1997; 54:745-751. 3. Savi P, Herbert JM, Pflieger AM, et al. Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol. 1992; 44:527-532. 4. American Society of Regional Anesthesia and Pain Medicine. Second Consensus Conference on Neuraxial Anesthesia and Anticoagulation. Consensus statements on neuraxial anesthesia and anticoagulation: Section on anesthetic management of the patient receiving antiplatelet medications. Available at http://www.asra.com/consensus-statements/2.html. Accessed February 2007. 5. Hoffman R, Benz EJ Jr, Shattil SJ, et al. Hematology: Basic Principles and Practice. 3rd ed. New York: Churchill Livingstone; 2000:2173. ITEM 5 Ketamine indirectly stimulates the cardiovascular system. A centrally mediated increase in sympathetic tone and circulating catecholamines produces an increase in heart rate, blood pressure, and cardiac output. Two other mechanisms—prevention of reuptake of norepinephrine into postganglionic sympathetic nerve endings and depression of baroreceptor reflexes—may contribute to the indirect hemodynamic efforts of ketamine. While some have advocated use of ketamine in hypotensive trauma patients, it must be recognized that ketamine is a direct myocardial depressant and administration to a patient who is already catecholamine-depleted may produce hypotension. Although epidural ketamine may produce a synergistic effect when administered in combination with opioids or local anesthetics, ketamine used alone produces minimal analgesia when administered via this route. Ketamine increases the cortical amplitude of somatosensory evoked potentials but decreases auditory and visual evoked responses. Although the mechanism is not clear, ketamine enhances the effect of nondepolarizing neuromuscular blocking drugs. A prolonged duration of apnea occurs when succinylcholine is administered in conjunction with ketamine. REFERENCES 1. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:172-173. 2. Crochetière C. Obstetric emergencies. Anesthesiol Clin North America. 2003; 21:111-125. ITEM 6 Mediastinoscopy is a procedure used to obtain lymph node biopsies and is performed by inserting a mediastinoscope above the level of the sternal notch (Figure 1). Complications that may occur include bleeding, pneumothorax, nerve injury, esophageal injury, infection, and compression of structures in the mediastinum including the trachea and the great vessels and their branches. Injury to the great vessels (eg, 8 pulmonary artery) poses a risk of life-threatening hemorrhage. The overall complication rate is reported to be 1.5% to 3%. Figure 1. Path of a mediastinoscope demonstrating the structural relationship of important anatomy in the mediastinum. Used with permission, from Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. Compression of the innominate artery may decrease blood flow to the right carotid and right subclavian arteries. Decreased right carotid artery blood flow may cause decreased cerebral blood flow with potential cerebral ischemia, especially in patients with preexisting cerebrovascular disease. Decreased right subclavian artery blood flow may cause loss of the right radial pulse and attenuation of the pulse oximetry signal when the probe is located on a right upper extremity digit. The superior vena cava may be compressed from the scope or injured during biopsy attempts, but this would not result in sudden loss of the right radial artery pulse. If significant bleeding is present from injury to this vessel, attempts should be made to establish intravenous access from below the diaphragm. The left subclavian vein and the descending aorta are not located in the region occupied by the mediastinoscope. Compression of the ascending aorta, trachea, or vagus nerve may lead to bradycardia or other dysrhythmias. 9 REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:19091911. 2. Hensing, TA. Clinical evaluation and staging of patients who have lung cancer. Hematol Oncol Clin North Am. 2005; 19:219-235. ITEM 7 Early diagnosis and use of dantrolene has reduced mortality associated with malignant hyperthermia (MH) from 70% to 5%. Release of tissue thromboplastin stimulated by acidosis, fever, hypoperfusion, and hypoxia may produce disseminated intravascular coagulation. Other complications of MH include cardiovascular o tachycardia o dysrhythmias o hypotension o decreased cardiac output o cardiac arrest pulmonary o hypoxemia o hypercarbia o pulmonary edema renal o oliguria o anuria o renal failure central nervous system o areflexia o cerebral edema o coma o fixed and dilated pupils Pericarditis and hepatitis (potentially fatal) are recognized complications of long-term oral dantrolene therapy, but they have not been reported in the acute treatment of patients with short-term use of intravenous dantrolene. Some patients receiving long-term oral dantrolene therapy (eg, patients with spasticity) in doses ranging from 100 mg/day to 400 mg/day have been reported to develop pleural effusions, but this complication has not been reported with short-term use of intravenous dantrolene. Other complications of long-term dantrolene therapy include hypothyroidism, aplastic anemia, heart failure, leukopenia, and lymphocytic lymphoma. After an acute episode of MH, the patient and immediate family members should have appropriate counseling and possible referral for determination of MH susceptibility. A letter to future anesthesia providers and Medic-Alert bracelet information should be provided to the patient. Anesthesia providers can consult an MH expert at the Malignant Hyperthermia Association of the United States (MHAUS). MHAUS also publishes patient and health care provider information. Refer to the MHAUS Website at www.mhaus.org or telephone the MH emergency hotline at 1-800-644-9737. 10 REFERENCES 1. Ali SZ, Taguchi A, Rosenberg H. Malignant hyperthermia. Best Pract Res Clin Anaesthesiol. 2003; 17:519-533. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:11841185. ITEM 8 Some complications (urinary tract infections, deep venous thrombosis, and pulmonary embolism) associated with paraplegia are more likely to occur in parturients. The respiratory changes of pregnancy, including decreased functional residual capacity and expiratory reserve volume, can increase the risk of pulmonary compromise in women with paraplegia. In parturients with a spinal cord lesion above T11, the pain of uterine contractions will not be felt, which could delay the diagnosis of preterm labor. Autonomic hyperreflexia is one of the most dramatic complications that may occur in laboring women with a chronic spinal cord injury. If steps are not taken to prevent this syndrome, approximately 85% of parturients with a lesion at T6 or higher will experience autonomic hyperreflexia during labor. Although it is significantly less common in patients with a lesion between T7 and T10, autonomic hyperreflexia has also been reported in these patients. Laboring women with a chronic spinal cord injury may experience autonomic hyperreflexia because central inhibition of the sympathetic neurons cannot occur below the level of the spinal cord injury. Noxious stimuli, including uterine contractions, cause afferent transmission via peripheral nerves to the dorsal horn of the spinal cord. The afferent neurons synapse with sympathetic neurons, which then traverse via the anterior roots to the paraspinal sympathetic chain. The sympathetic impulse is propagated cephalad and caudad within the sympathetic chain, leading to significant sympathetic hyperactivity. Vasoconstriction below the level of the spinal cord injury produces severe hypertension. Baroreceptor responses to this hypertension in the aortic and carotid sinus result in vasodilatation above the level of the spinal cord lesion as well as bradycardia. However, with higher spinal cord injuries the compensatory vasodilatation is inadequate to prevent the severe hypertension. Signs and symptoms of autonomic hyperreflexia include: cyclic hypertension (substantial increase in blood pressure during contractions followed by a return to baseline between contractions) bradycardia blurred vision facial flushing nasal congestion profuse swelling above the level of spinal cord injury increased cutaneous temperature above the level of injury Methods to prevent autonomic hyperreflexia should be instituted early in labor or before labor induction is begun. Epidural analgesia with a local anesthetic is the most common technique used to both prevent and treat this syndrome. Successful prevention has been reported with the use of a higher concentration of local anesthetic (eg, 0.25% bupivacaine or higher). It can be difficult to assess the sensory level that has been achieved with epidural analgesia in a paraplegic patient, but assessment of segmental reflexes below the level of the spinal cord injury is useful. The administration of epidural fentanyl alone to provide labor analgesia has not been successful at preventing the development of autonomic hyperreflexia. Although a woman with a T5 spinal cord injury will not experience labor pain, epidural labor analgesia with a higher 11 concentration of local anesthetic (eg, at least 0.25% bupivacaine) still must be provided to eliminate the risk of autonomic hyperreflexia. Paraplegia is not considered an absolute indication for cesarean delivery. Vaginal delivery is preferred unless an obstetric indication for surgical delivery is present. Because the paraplegic parturient is unable to push, an instrument-assisted vaginal delivery is often performed. REFERENCES 1. Chestnut DH. Obstetric Anesthesia: Principles and Practice. 3rd ed. Philadelphia: Elsevier Mosby; 2004:875-877. 2. Crosby E, St.-Jean B., Reid D, et al. Obstetrical anaesthesia and analgesia in chronic spinal cordinjured women. Can J Anaesth. 1992; 39(5 Pt 1):487-494. ITEM 9 Although some professional and Olympic athletes use performance-enhancing drugs, they select drugs that are difficult to detect such as erythropoietin or growth hormone. In contrast, amateur athletes, body builders, and security professionals are much more likely to choose anabolic steroids. It is estimated that 15%-40% of regular users of exercise facilities or health clubs have used anabolic steroids, which means that on any given day about 3 millions individuals may be taking anabolic steroids. Current illegal steroids have predominantly anabolic not androgenic effects. Steroids are used to enhance exercise tolerance by protecting muscle fibers from damage during stress and increasing protein synthesis for postactivity recovery. Side effects vary by the steroid chosen but commonly reported ones include: increased libido (61%) changes in mood (48%) reduced testicular volume (46%) acne (43%) gynecomastia (19%) abnormal liver function and electrolytes (% unknown) Minor side effects include striae, male pattern baldness, and hirsutism, particularly in women and adolescents. Overall risk of mortality is approximately four times greater in steroid users than nonusers. Common associated fatal diseases are from premature cardiac (eg, myocardial infarction) and neoplastic (eg, renal, hepatic, and testicular) disease. Physicians often are not suspicious of these comorbid conditions due to the apparent health and age of the patient. Adverse cardiovascular effects include hypertension, left ventricular hypertrophy, diastolic dysfunction, polycythemia, and spontaneous thrombosis secondary to a hypercoagulable state. Polycythemia and a hypercoagulable state make these patients at greater risk than nonusers of steroids for coronary, cerebral, peripheral, and deep vein thrombosis (DVT). Consequently, DVT prophylaxis is very important in the perioperative period. The increased ventricular size and focal areas of fibrosis are associated with sudden death during exercise. High circulating lipids increase in the incidence of atherosclerosis. About 88% of chronic users report one or more withdrawal symptoms such as reduced muscle size, weakness, fatigue, depression, or decreased libido when discontinuing anabolic steroids. There are case reports of intractable weakness requiring prolonged postoperative ventilation after major surgery. 12 According to one case report, administration of anabolic steroids was necessary to treat severe muscle weakness. Unusual perioperative problems can include compartment syndromes especially in lithotomy or seated positions or following trauma. Poor cardiac function, particularly diastolic dysfunction, predisposes steroid abusers to congestive heart failure with generous fluid administration. Due to the large muscle mass and increased total body water, an increased dosage of nondepolarizing muscle relaxants is necessary to provide adequate intubating conditions and maintain muscle relaxation. Oral anabolic steroids act as hepatic enzyme inducers; appropriate adjustment in drug dosing must be made. Mineralocorticoid effects can lead to electrolyte imbalance that is often treated with self-prescribed diuretics. Nonjudgmental inquiry into anabolic steroid use is important especially for anesthesiologists who frequently provide care for amateur and professional athletes. REFERENCES 1. Kam PC, Yarrow M. Anabolic steroid use: Physiological and anaesthetic considerations. Anaesthesia. 2005; 60:685-692. 2. Kutscher EC, Lund BC, Perry PJ. Anabolic steroids: A review for the clinician. Sports Med. 2002; 32:285-296. 3. Ford M, Delaney KA, Ling L, Erickson T. Clinical Toxicology. Philadelphia: WB Saunders; 2001:595-600. 4. Evans NA. Current concepts in anabolic-androgenic steroids. Am J Sports Med. 2004; 32:534542. ITEM 10 Local anesthetics can cause a range of local and systemic symptoms. However, prospective studies suggest that very few of these adverse reactions are confirmed as true allergic reactions. Allergy to the ester local anesthetics (procaine, tetracaine, chloroprocaine) is known and probably the result of cross sensitivity with sulfonamides. These drugs are derivatives of p-aminobenzoic acid (PABA), a substance known to be allergenic. Amide local anesthetics (bupivacaine, lidocaine, mepivacaine) are not derivatives of PABA and true allergic reactions are extremely rare. Occasionally, solutions of amide local anesthetics may contain a preservative, methylparaben, which has a chemical structure similar to that of PABA and may induce an allergic reaction. Additionally, some vials use latex as a sealant and have been implicated in some presumed local anesthetic allergies. Most adverse reactions attributed to allergic reactions are caused by intravascular injections or rapid absorption of an excess of local anesthetic. European investigators reported that of a group 141 adult patients alleged to have a local anesthetic “allergy” all failed to demonstrate an allergic reaction on skin testing. Additionally, in a subgroup of 44 patients, an intra-oral challenge also failed to elicit an allergic response. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:596597. 13 2. Rood JP. Adverse reaction to dental local anaesthetic injection—‘allergy’ is not the cause. Br Dent J. 2000; 189:380-384. 3. Sidhu SK, Shaw S, Wilkinson JD. A 10-year retrospective study on benzocaine allergy in the United Kingdom. Am J Contact Dermat. 1999; 10:57-61. ITEM 11 Alveolar ventilation regulates the intake of the volatile anesthetic into the lungs. Hyperventilation causes increased delivery of volatile agents to the lungs and results in a decreased amount of time for alveolar partial pressure to approximate inspired partial pressure. Hypoventilation results in a slower rate of increase of alveolar concentration and slower induction time for anesthesia. Increased cardiac output results in increased blood flow through the lungs with resulting increased uptake of volatile anesthetic from the alveoli into the blood stream; this will delay the increase in alveolar concentration of the volatile anesthetic. A decreased cardiac output results in decreased blood flow to the lungs and decreased uptake of the volatile agent by the blood; this decreases the time it takes for the alveolar concentration to approach the inspired concentration of a volatile anesthetic. The blood:gas partition coefficient is a factor for determining the transfer of volatile anesthetics from the alveoli to arterial blood. This coefficient is used to describe the volatile agent’s solubility compared into two phases: the blood and gas. An increased blood:gas partition coefficient will result in increased uptake of more volatile agent from the alveoli into the bloodstream, thereby delaying the increase in alveolar concentration of the volatile anesthetic. The vessel-poor (VPG) is a group of tissues made up of bone, tendons, ligaments, and cartilage. The VPG receives negligible perfusion; as a result, changes in the volume of the VPG do not appreciably contribute to the transfer of volatile anesthetics. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:131139. 2. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:24-31. 3. Eger EI 2nd, Saidman LJ. Illustrations of inhaled anesthetic uptake, including intertissue diffusion to and from fat. Anesth Analg. 2005; 100:1020-1033. ITEM 12 Bacterial meningitis is a rare but serious complication of dural puncture. Anesthesiologists should be aware of the signs and symptoms as well as epidemiology of this disease. An analysis of 180 cases of postdural puncture bacterial meningitis reported in the medical literature since 1952 provides information for practitioners. It is important to realize, however, that the value of these data is limited because the study is based only on the case reports that have been published and does not include a denominator. Among the reported cases of bacterial meningitis, the distribution of procedures performed is found in Table 1. Over 70% of cases involved neuraxial anesthesia with the majority of cases occurring after spinal anesthesia. 14 Table 1. Distribution of procedures resulting in bacterial meningitis. Adapted from Baer ET. Post-dural puncture bacterial meningitis. Anesthesiology. 2006; 105:381-393. Procedure Meningitis Cases (%) Spinal anesthesia 96 (54) Epidural anesthesia 22 (12) Combined spinal-epidural anesthesia 10 (6) Myelography 29 (16) Diagnostic lumbar puncture 17 (9) Pneumoencephalography 2 (1) Epidural steroid injection 2 (1) Not indicated 2 (1) Postdural puncture bacterial meningitis presents with the same signs and symptoms as other cases of bacterial meningitis. These include headache neck pain fever vomiting nuchal rigidity altered mental status Some of these findings, especially headache and neck pain, are associated with spinal headache, a much more common complication of dural puncture. However, the presence of fever or altered mental status should alert the anesthesiologist to the possible diagnosis of meningitis. Most cases of bacterial meningitis develop 6-36 hours after the dural puncture. Prompt diagnosis and treatment is essential to improve outcome. Among the 180 cases that were analyzed in a recent review, three deaths were reported. All of these deaths occurred in obstetric patients. The diagnosis of postdural puncture bacterial meningitis was not considered in any of these patients when they first presented with typical symptoms of headache and fever. 15 Organisms from the mouth and upper airway of medical personnel are the most common organisms identified in bacterial meningitis following dural puncture, suggesting that the source of infection is droplet contamination. The distribution of organisms isolated from the 180 patients in the recent review supports this mechanism of infection. The most frequently identified organisms, found in 49% of patients, were viridans group streptococci, which are common oral bacteria. In 76% of cases, flora that are present in the mouth or upper airway were identified. Other data also support droplet contamination as the most common cause of postdural puncture bacterial meningitis. A study in Sweden that investigated neurologic complications following neuraxial anesthesia reported that 97% of meningitis cases were caused by viridans streptococcus. In two published cases, the cerebrospinal fluid isolates of patients who developed meningitis after neuraxial procedures matched the nasal or pharyngeal isolates of the physicians who performed the procedures. Although droplet contamination may be the most common etiology of bacterial meningitis after dural procedure, other causative mechanisms have been identified. These include contamination by skin bacteria and, infrequently, an endogenous bacterial source, such as bacteremia. REFERENCES 1. Baer ET. Post-dural puncture bacterial meningitis. Anesthesiology. 2006; 105:381-393. 2. Chestnut DH. Obstetric Anesthesia: Principles and Practice. 3rd ed. Philadelphia: Elsevier Mosby; 2004:655. 3. Hepner DL. Gloved and masked—will gowns be next? The role of asepsis during neuraxial instrumentation. Anesthesiology. 2006; 105:241-243. ITEM 13 A 79-year-old man with acute myelogenous leukemia is intubated and ventilated in the intensive care unit. He has multiple organ failure and his leukemia has not responded to treatment. He is comatose. Based on his previously stated wishes, his family wants to withdraw support and provide “comfort care only.” The patient’s trachea is extubated and he breathes at a rate of 35 breaths per minute and appears to be uncomfortable and gasping for air. An infusion of fentanyl is commenced and lorazepam is administered as required, both drugs being titrated to a respiratory rate of 10-12 breaths per minute and to patient comfort. Two hours later, the patient dies. One of the family members asks if the drugs were used to end the patient’s life. One of the major approaches to medical ethics, proposed by Beauchamp and Childress, has been labeled principalism. The approach proposed four major principles: autonomy, beneficence, nonmaleficence, and justice. Autonomy implies a respect for the patient’s right to self-determination. Autonomy implies that the patient has decision-making capacity (the right to refuse medical therapy, even at the risk death). A decision to withhold or proceed with life-sustaining treatment in the event of a permanent state of unconsciousness may have been made by a patient. If the patient was previously capable of making decisions and the decision was clear and unequivocal, the patient’s wish should be respected unless it was subsequently clearly rescinded. The decision to withdraw life support in this case was based on the principle of autonomy. Decisions that bypass or override the patient’s autonomous decision making are described as paternalistic or parentalistic. Such physician behavior is justified only in certain circumstances: 16 when there exists a risk of significant, preventable harm to the patient the harm will be prevented by the paternalistic action the projected benefits outweigh the risks to the patient the action that least restricts the patient’s autonomy is used In this case, the patient’s wishes were carried out. Abandonment is the practice of leaving a patient (for whom the physician has provided health care in the past) without providing for immediate or future medical care. Once a physician and patient mutually agree to enter into a therapeutic relationship, the physician has a duty to provide ongoing medical care. In this case, the physician continued a professional relationship with the patient. The tenets of beneficence and nonmaleficence play a role in the concept of “double effect.” Beneficence is the good faith act on the physician’s part to preserve life, relieve suffering, restore health, and restore or maintain function. The principle of nonmaleficence is based on “do no harm, prevent harm, and remove harm.” In the circumstance where sedative and analgesic medications are administered to relieve suffering (a beneficent outcome) there is a risk of an adverse outcome (suppression of respiration to the point of causing death from respiratory arrest). The rule or principle of double effect is an attempt to resolve this conflict: the act itself must be good or morally neutral the person performing the act should desire only the good effect the good effect must not be produced by means of the adverse effect the beneficial effect must outweigh the adverse effect In this case, the sedatives and analgesics were given to relieve pain and suffering and not to hasten the patient’s death. Adequate analgesia, particularly in patients with incurable disease, is the physician’s responsibility, and the physician has not behaved immorally if death in a terminally ill patient is a result of respiratory depression from analgesic therapy. Euthanasia was not the goal. REFERENCES 1. Hook CC, Prakash UBS, Dunn WF. Medical ethics. In: Prakash UBS, Habermann TM. Mayo Internal Medicine Board Review 2000-01. Philadelphia: Lippincott Williams & Wilkins; 2000:607-623. 2. Truog RD, Cist AF, Brackett SE, et al. Recommendations for end-of life care in the intensive care unit: The Ethics Committee of the Society of Clinical Care Medicine. Crit Care Med. 2001; 29:2332-2348. ITEM 14 The sodium channel blockers are a class of drugs that are successfully used in the treatment of neuropathic pain. Lidocaine is a sodium channel blocker that is administered intravenously. The clinical use of systemic lidocaine for pain management was first reported in 1961 by anesthesiologists who noted its efficacy in postoperative pain. Over the last 40 years, systemic lidocaine has been used effectively for a variety of neuropathic pain conditions including diabetic neuropathy, central neuropathic pain, perioperative pain, phantom limb pain and stump pain, complex regional pain syndrome, traumatic peripheral neuropathies, postherpetic neuralgia, and others. Systemic lidocaine has gained much attention partly because animal models have demonstrated a link between injured nerves and spontaneous ectopic neural activity that can be suppressed with systemic lidocaine in clinically relevant doses. Additional interest in the use of 17 systemic lidocaine comes from the recognition that the oral lidocaine congener mexiletine can provide a reasonable long-term management strategy. Carbamazepine, oxcarbazepine, and topiramate are examples of oral antiepileptic agents that have sodium channel blocking properties and are commonly used to treat neuropathic pain. None of these drugs have Food and Drug Administration approval for the treatment of neuropathic pain. Although the primary mechanism of action is intravenous (IV) lidocaine is postulated to be the inhibition of firing of action potentials by blocking sodium channels, this is thought to be an overly simplistic view. Lidocaine has been found to act on many different channels (eg, inactivation of calcium channels) and receptors (eg, inactivation of G-protein-coupled receptors). Furthermore, the plasma lidocaine concentrations that relieve pain are far below those that block normal action potentials. This and other evidence suggests that the actual mechanisms of IV lidocaine are multiple. While IV lidocaine has not been shown one way or another to interact with the gamma-aminobutyric acid (GABA) receptor, antagonism of the GABA receptor would not be expected to provide analgesia for neuropathic pain. Although lidocaine inactivates G-proteincoupled receptors, this is not thought to be its primary analgesic mechanism. REFERENCES 1. Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000; 87:7-17. 2. Kastrup J, Petersen P, Dejgard A, et al. Intravenous lidocaine infusion—a new treatment of chronic painful diabetic neuropathy? Pain. 1987; 28:69-75. 3. Wallace MS, Ridgeway BM, Leung AY, et al. Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. 2000; 92:75-83. 4. Attal N, Rouaud J, Brasseur L, et al. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology. 2004; 62:218-225. ITEM 15 A 65-year-old man undergoes placement of an epidural spinal cord stimulator for treatment of severe pain caused by inoperable peripheral vascular disease. Following placement, he has a marked decreased in the severity of the pain. Epidural spinal cord stimulation (SCS) has been used successfully to treat neuropathic pain worldwide. It is also used extensively in Europe and to a lesser extent in North America for treatment of angina and pain associated with peripheral vascular disease. Several theories have been proposed to explain the mechanism of action of SCS in peripheral vascular disease. One theory suggests that antidromic stimulation of the spinal cord results in release of mediators (substance P, prostacyclin, calcitonin gene-related protein) resulting in vasodilation. A second hypothesis is that SCS inhibits spino-cortico transmission of nociceptive signals. A third theory is that SCS directly inhibits the sympathetic nervous system, thereby directly inhibiting peripheral vasoconstriction. A fourth theory suggests that antidromic stimulation of unmyelinated C fibers or thinly myelinated A-delta fibers causes peripheral vasodilation. While it still requires elucidation, the most likely mechanism by which SCS works for patients with PVD is through a decrease in sympathetic activity. This results in peripheral vasodilation with a subsequent decrease in pain. The vasodilation may help with wound healing. 18 There is no evidence that SCS results in an enhanced release of glutamate. Enhanced activity of glutamate, an excitatory amino acid, would be expected to result in an increase in pain. Many pain treatments are aimed at reducing glutamate release in the spinal cord. A-alpha fibers are involved in muscle efferents. Stimulation of these fibers would result in muscle contraction and would not be desirable in SCS. When they are stimulated, it is often a sign that the SCS lead has moved to the anterior epidural space. There is no evidence to support direct stimulation of opioid receptors as a mechanism of action in SCS. REFERENCES 1. Loeser JD, Butler SH, Chapman CR, et al. Bonica’s Management of Pain. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2001:30, 236-239. 2. Erdek MA, Staats PS. Spinal cord stimulation for angina pectoris and peripheral vascular disease. Anesthesiol Clin North America. 2003; 21:797-804. 3. Tanaka S, Barron KW, Chandler MJ, et al. Role of primary afferents in spinal cord stimulationinduced vasodilation: Characterization of fiber types. Brain Res. 2003; 959:191-198. ITEM 16 Liver disease may present significant problems for the anesthesiologist, depending on the severity of the patient’s illness. End-stage liver disease due to cirrhosis becomes a multisystem disease with potential cardiac, pulmonary, renal, neurologic, and other organ system involvement. The treatment of choice for end-stage liver disease is orthotopic liver transplantation. The Child-Turcotte-Pugh score (Table 1) has been used for many years to quantify the severity of liver dysfunction and the Child-Turcotte-Pugh classification was one of the criteria used to prioritize patients for liver transplantation. Table 1. Child-Turcotte-Pugh classification. Class A = 1-6 points, Class B = 7-9 points, Class C = 10-15 points. Encephalopathy grade Ascites Bilirubin (mg/dL) Albumin level (g/dL) Prothrombin time (seconds prolonged) 1 Point 2 Points 3 Points None 1, 2 3, 4 Absent Slight Moderate 1-2 2-3 >3 > 3.5 2.8-3.5 < 2.8 <4 4-6 >6 It was increasingly recognized that the Child-Turcotte-Pugh score was a suboptimal measure of liver dysfunction. For example, a patient with a bilirubin of 40 mg/dL received the same number of points as a patient with a bilirubin of 4 mg/Dl, though the former patient is more ill. In addition, some of the ChildTurcotte-Pugh criteria were subjective (eg, degree of encephalopathy) or open to institutional differences (eg, prothrombin time). Accordingly, a new system for determining the severity of liver dysfunction was required. Hence, the Model for End-stage Liver Disease (MELD) was introduced and validated. 19 The MELD score is based solely on the patient’s bilirubin, creatinine, and international normalization ratio (INR). The higher a patient’s MELD score, the more likely he or she is to die within three months. In February 2002 the United Network for Organ Sharing (UNOS), the donor organ allocation body in the United States, adopted the MELD score as the criterion for determination of priority for liver transplantation. There are two variations on the MELD score—one is used to determine the need for a transjugular intrahepatic portosystemic shunt (TIPS), the other is used as a score for liver transplantation. The only difference between the two equations is the exclusion of the factor relating to cause of liver disease when evaluating the patient for liver transplantation. Both the MELD score and the Child-Turcotte-Pugh classification were originally developed to predict the outcome of patients after transjugular intrahepatic portosystemic shunts. The MELD score was derived from prospective data whereas the Child-Turcotte-Pugh classification was derived from empiric data. The variables in the MELD score are objective, not subject to institutional differences, and lack the ceiling effect of the Child-Turcotte-Pugh classification. REFERENCES 1. Keegan MT, Plevak DJ. Pre-operative assessment of the patient with liver disease. Am J Gastroenterol. 2005; 100:2116-2127. 2. Stoelting RK, Dierdorf SF. Anesthesia and Co-Existing Disease. 4th ed. New York: Churchill Livingstone; 2002:299-324. 3. Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: Application of survival models to liver allocation. Liver Transpl. 2001; 7:567-580. ITEM 17 Massive transfusion is defined as the replacement of the patient’s total blood volume in a 24-hour period. The complications associated with large volume blood transfusion include dilutional coagulopathy, disseminated intravascular coagulation, fibrinolysis, citrate toxicity, hyperkalemia, hypocalcemia, acidbase imbalance, impaired hemoglobin function, and hypothermia. Each unit transfused also increases the risk of transmission of infections such as hepatitis and HIV, though the absolute risk of acquiring such infections from blood transfusion is low because of screening of donor units. Citrate, used as a preservative in banked blood, chelates calcium, resulting in hypocalcemia. The hypotension with narrow pulse pressure occurring as a consequence of hypocalcemia may initially be difficult to distinguish from hypovolemia, but hypocalcemia is associated with elevated rather than depressed ventricular filling pressures. These signs do not manifest unless approximately one unit of blood is given every five minutes to an average 70 kg person. Hypocalcemia may also impair clotting function. In the setting of large transfusions, serum calcium should be measured and hypocalcemia treated accordingly. After24-48 hours of storage, platelet activity is decreased to about 5%-10% of normal. Damaged platelets are removed by the reticuloendothelial system soon after transfusion. Thus, dilutional thrombocytopenia will occur and may be a cause of hemorrhagic diathesis in a patient who has received multiple units of banked blood. It is recommended that the platelet count be maintained above 75-100 x 109/L in the setting of massive transfusion. The oxygen-hemoglobin dissociation curve is shifted to the left because 2,3-diphosphoglycerate is decreased in banked blood. Hypothermia and alkalosis can accentuate this shift, making tissue hypoxia a 20 theoretical possibility. However, tissue hypoxia from decreased 2,3 DPG is not considered a significant clinical problem as levels return to normal within hours after transfusion of red blood cells. Infusion of large amounts of relatively cold blood products (4°C) will usually cause the patient’s body temperature to decrease, which can itself cause coagulopathy. Efforts must be made to warm the fluids as they are being administered, in addition to the usual techniques of maintaining patient normothermia. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:17991830. 2. Faust RJ. Anesthesiology Review. 3rd ed. New York: Churchill Livingstone; 2002:456-457. ITEM 18 Though uncommon, infective endocarditis is life-threatening. Some controversy exists regarding the cost effectiveness of endocarditis prophylaxis, but administration of prophylactic antibiotics is the standard of care for patients with specific conditions undergoing certain procedures. The American Heart Association published recommendations for administration of prophylactic antibiotics in 1997. Consideration must be given to both the nature of the cardiac lesion and the nature of the surgical or nonsurgical procedure to be performed. Patients with cardiac lesions are divided into those whose lesions post high, moderate, or negligible risk for infective endocarditis. Patients in the high-risk category include those with prosthetic cardiac valves, including bioprosthetic and homograft valves previous bacterial endocarditis complex congenital cyanotic heart disease surgically constructed systemic pulmonary shunts or conduits Patients at moderate risk are those with acquired valvular dysfunction hypertrophic cardiomyopathy mitral valve prolapse with valvular regurgitation and/or thickened leaflets other lesions not mentioned in the high-risk or negligible-risk categories Lesions that do not require endocarditis prophylaxis include isolated secundum atrial septal defect surgically repaired atrial septal defect, ventricular septal defect, or patent ductus arteriosus (without residua and beyond six months) previous coronary artery bypass graft surgery physiological, functional, or innocent heart murmurs previous Kawasaki disease without valvular dysfunction previous rheumatic fever without valvular dysfunction cardiac pacemakers and implantable defibrillators 21 Endocarditis prophylaxis is NOT recommended for the following procedures: respiratory o tracheal intubation o flexible bronchoscopy with or without biopsy* o tympanostomy tube placement gastrointestinal o transesophageal echocardiography o endoscopy with or without gastrointestinal biopsy* genitourinary tract o vaginal hysterectomy* o cesarean delivery o in uninfected tissue urinary catheterization uterine dilation and curettage therapeutic abortion sterilization procedures insertion or removal of intrauterine devices other o cardiac catheterization, including balloon angioplasty o implanted cardiac pacemakers, implantable defibrillators, and coronary stents o incision or biopsy of surgically scrubbed skin o circumcision o cataract surgery *Prophylaxis is optional for patients with high-risk lesions. REFERENCES 1. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. 197; 277:1794-1801. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:10791081. 3. American Heart Association. Endocarditis. Available online at: http://www.americanheart.org/presenter.jhtml?identifier=3004539. Accessed February 2007. ITEM 19 A new brand of tracheal tube is purchased by the hospital. Over the next month, four patients experience unexplained tracheal tube obstruction requiring tracheal tube replacement under difficult circumstances. Postmarking adverse event reporting is the major method by which the Food and Drug Administration (FDA) can detect unforeseen equipment and device failures as well as adverse drug actions. Unexpected problems may be so infrequent that individuals or practice groups cannot recognize the pattern of failure or the global implications. Examples in which postmarking reporting have lead to product recall include drugs (eg, rapacuronium) and devices (eg, pacemakers leads). Changing the brand of such common devices as a tracheal tube or central line catheter can result in an unexpected change in functional use, leading to patient injury. Even minor component changes in the manufacture of a medical device (eg, using a different source for plastic or switching to an alternative computer component) can adversely affect performance. 22 Reporting of all adverse events suspected to be associated with the performance of a device, equipment, or drug is mandatory for all hospitals and physicians. MedWatch, an FDA website (http://www.fda.gov/medwatch/) provides instructions on how to report a suspected adverse event. This activity can be completed online (https://www.accessdata.fda.gov/scripts/medwatch/medwatchonline.htm), or forms can be printed and returned by fax or mail. Reporting also can be completed by telephone. The FDA systematically reviews the information submitted and issues alerts/warnings and recalls. Health care providers can subscribe to email newsletters on 20 or more topics. Health professionals and health educators have a website designed to provide specific information. The FDA maintains a database where reports of problems are available. Prior to purchase of a produce it is reasonable to check the FDA website for performance problems and ask the manufacturer about ones that may be troubling in your environment. Similarly, reports of adverse events associated with an off-label drug use can be found here. It is important that practitioners recognize when they engage in off-label drug use or unsupported device use. Manufacturers are required and medical publications are expected to advise health care professionals when uses of drugs or devices are not FDA approved. With regard to the clinical scenario presented in this item, the FDA must be informed when there is a suspicion by any physician of a device malfunction. While any physician can easily complete the report, it is usually done by the quality assurance officer designated by the anesthesia group or hospital. While not required, other steps that can help prevent additional patient morbidity include a process that informs other health care providers within the institution who may use the drug, device, or equipment of the nature of the problem informs the product manufacturer institutes a reporting process to monitor for further failures considers voluntary replacement or removal of the product from use until the issue is resolved REFERENCES 1. Chang NS, Simone AF, Schultheis LW. From the FDA: What’s in a label? A guide for the anesthesia practitioner. Anesthesiology, 2005; 103:179-185. 2. US Food and Drug Administration website: http://www.fda.gov/default.htm. Accessed February 2007. 3. Katz RI, Lagasse RS. Factors influencing the reporting of adverse perioperative outcomes to be a quality management program. Anesth Analg. 2000; 90:344-350. ITEM 20 Any new-onset neurologic complaint after a spinal anesthetic causes great concern. Cauda equina syndrome can occur after a spinal anesthetic, epidural anesthetic, or spinal tap and is characterized by lower extremity weakness bowel and bladder dysfunction pain localized to the area of needle placement onset several hours to days after the neuraxial needle placement Patients suspected of having cauda equina syndrome should undergo magnetic resonance imaging (MRI) to detect collected fluid that is compressing spine contents. Permanent paralysis is likely even with rapid surgical decompression. Motor and sensory changes progress rapidly. Other causes of progressive neurologic deterioration include spinal cord hematoma or abscess, spinal cord ischemia due to 23 hypotension or anemia, mechanical injury such as disc herniation, or intrathecal administration of neurotoxic drugs. The symptoms presented here are much more consistent with transient neurologic symptoms (TNS). Patients with TNS characteristically present with pain in the gluteal area that radiates down both legs onset approximately 24 hours after spinal anesthetic no other neurological symptoms Without progressive neurologic symptoms an immediate MRI is not required. Treatment consists of reassurance and an oral analgesic. The symptoms resolve gradually and rarely last more than 10 days. Progressive neurologic deterioration does not occur. The frequency of TNS may be as high as one in seven depending on the drug administered. REFERENCES 1. Zaric D, Christiansen C, Pace NL, et al. Transient neurologic symptoms after spinal anesthesia with Lidocaine versus other local anesthetics: A systematic review of randomized, controlled trials. Anesth Analg. 2005; 100:1811-1816. 2. Fleisher LA. Evidence-Based Practice of Anesthesiology. Philadelphia: Elsevier Saunders; 2004:370-371. ITEM 21 Parturients with a placenta previa and a history of cesarean delivery are at increased risk for placenta accreta. Placenta accreta refers to an abnormal placentation in which the placenta abnormally adheres to the myometrium, making separation difficult. Three types of placenta accreta occur (Figure 1): placenta accreta, where the placenta is adherent to the myometrium but does not invade it placenta increta, where the placenta invades the myometrium placenta percreta, where the placenta invades through the myometrium to the serosa and my invade into other pelvic structures, most commonly the bladder 24 Figure 1. Classification of placenta accreta, increta, and percreta. From Birnbach DJ, Gatt SP, Datta S [eds]: Textbook of Obstetric Anesthesia. New York, Churchill Livingstone, 2000:406. Originally from Kamani AAS, Gambling DR, Christilaw J, et al. Anesthetic management of patients with placenta accreta. Can J Anaesth. 34:613, 1987. Data from Miller DA, Chollet JA, Goodwin TM. Clinical risk factors from placenta previa-placenta accreta. Am J Obstet Gynecol. 1997; 177:210. Used with permission from Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:Figure 58-18. Various studies have reported placenta accreta in 10%-25% of women with placenta previa and one prior cesarean delivery. The risk significantly increases in parturients with a history of two or more cesarean deliveries. Investigators have found that approximately 50%-60% of those patients develop this abnormal placentation (Figure 2). 25 Figure 2. Relationship between the number of previous cesarean sections and the incidence of placenta accreta in patients with placenta previa. (From Clark SL, Koonings PP, Phelan JP, et al: Plaenta previa/accreta and prior cesarean section. Obstet Gynecol. 66:89, 1985; and Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-accreta. Am J Obstet Gynecol. 177:210, 1997.). Used with permission from, Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:Figure 58-19. Establishing the diagnosis of placenta accreta in the antepartum period is difficult. Ultrasonography and magnetic resonance imaging are sometimes useful in identifying the abnormality. However, the predictive value for either of these imaging techniques is poor. Postpartum hemorrhage may be a serious complication of placenta accreta, especially when the diagnosis is not suspected. Attempts to remove the adherent placenta can lead to rapid and massive blood loss. Once the diagnosis is confirmed, recommended obstetric management usually includes prompt hysterectomy. Major blood loss often occurs during the hysterectomy, even when removal of the placenta has not been attempted. Before proceeding with cesarean delivery in a patient at risk for placenta accreta, it is essential that a type and cross match be performed and blood be available in the operating room. Other preparations for massive transfusion should be made, such as obtaining adequate large-bore venous access. The anesthesiologist might also consider intraoperative blood salvage. Use of this technique during obstetric procedures is controversial, however, because of concerns that amniotic fluid embolism could occur. To minimize contamination of the salvaged blood with fetal debris, a leukocyte depletion filter should be added to the blood washing system. Despite the use of this filter, fetal cells do occur within the salvaged blood. Some small studies of intraoperative blood salvage during cesarean delivery have reported no cases of amniotic fluid embolism but most experts believe maternal safety with this technique remains uncertain. Its use is only recommended when massive blood loss occurs. If blood salvage is utilized during cesarean delivery, collection of blood for transfusion should only being after the fetus and placenta have been delivered. 26 In a parturient at risk for placenta accreta, rapid and substantial blood loss may occur. In addition, surgery may be prolonged. Therefore, single-injection spinal anesthesia would not be a preferred anesthetic technique. Many anesthesiologists favor the use of general anesthesia for cesarean delivery when the potential for large blood loss exists, although epidural anesthesia has been used successfully in the setting of placenta accreta requiring hysterectomy. Preoperative fibrinogen levels may be useful in patients with abruptio placentae, but not in this patient. REFERENCES 1. Chestnut DH. Obstetric Anesthesia: Principles and Practice. 3rd ed. Philadelphia: Elsevier Mosby; 2004:673-674. 2. Crochetière C. Obstetric emergencies. Anesthesiol Clin North America. 2003; 21:111-125. ITEM 22 As the population ages, more and more older adults (> 65 years) will receive anesthetic care. Because of media coverage and the anecdotal experiences of friends and loved ones, many patients are very frightened by the possibility of permanent or even transient cognitive changes in the postoperative period. Postoperative cognitive decline (POCD) is defined as changes in learning, coordination, or memory on one or more neuropsychological test. The incidence of POCD after noncardiac surgery is reported to be between 7% and 26%. The incidence of delirium, which is defined as acute confusion and difficulty with attention and consciousness, is between 10% and 60%. Many studies do not provide long-term follow-up to determine if the mental status changes observed are reflective of a short-lived delirium or a long-term cognitive decline. The most important risk factor for either condition is preexisting cognitive impairment. The incidence of both conditions increases with age, depression, and poor functional living status (eg, dependent living situations). A wide range of comorbid conditions are associated with delirium: abnormal blood pressure heart failure on admission abnormal electrolytes or glucose self-reported alcohol abuse opioid use before admission This information can be used to advise patients and families of the patient’s risk. Perioperative events associated with increased frequency of delirium include: type of surgical procedure (thoracic, aortic aneurysm, bilateral knee replacement) blood loss postoperative pain hematocrit below 30% blood transfusion electrolyte and metabolic derangements (ie, abnormal glucose, hyponatremia) 27 Risk factors for POCD include a prolonged anesthetic respiratory complications infection repeat operation within one week The risk factors that are within the control of the anesthesiologist include the patient’s anesthetic management and postoperative analgesia care. Surprisingly, the type of anesthetic, either general or regional, has not been demonstrated to change the risk of either POCD or delirium. Even more surprising, the data do not support intraoperative cardiovascular events, hypotension, bradycardia, or tachycardia as risk factors that increase the frequency of either complication. Administration of meperidine for postoperative analgesia is associated with a higher risk of postoperative delirium than use of either morphine or fentanyl. This difference has been hypothesized to be related to the anticholinergic properties of normeperidine. REFERENCES 1. Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: A systematic review. Anesth Analg. 2006; 102:1255-1266. 2. Rasmussen LS, Johnson T, Kuipers HM, et al. Does anaesthesia cause postoperative cognitive dysfunction? A randomized study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003; 47:260-266. 3. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: A meta-analysis of randomized trials. Br J Anaesth. 2000; 84:450-455. 4. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1402. ITEM 23 The possibility of hypoxemia, hypercarbia, and hypoglycemia should be initially assessed in any patient with postoperative confusion or agitation. Central anticholinergic syndrome should be considered, especially in elderly patients who have received scopolamine. Central anticholinergic syndrome can be caused by a long list of medications including antihistamines, antiparkinsonian drugs, antipsychotic agents, and tricyclic antidepressants. Some over-the-counter pharmaceuticals including sleeping aids (eg, Sominex, Sleep-Eze, and Compoz) and bronchodilators (eg, Asthma-Dor) as well as certain plants (eg, deadly nightshade, bittersweet, potato leaves and spouts, jimson weed, and coca) are capable of producing central anticholinergic syndrome. Although methadone and meperidine can also include this phenomenon, belladonna alkaloids (atropine and scopolamine) are probably the most common perioperative drugs associated with central anticholinergic syndrome. Elderly patients are particularly susceptible to the central anticholinergic syndrome, which presents with central nervous system manifestations (unconsciousness, somnolence, stupor, restlessness, delirium, hallucinations) as well as peripheral manifestations including fever, tachycardia, dry mouth, mydriasis, and urinary retention. 28 REFERENCES 1. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004; 22:389-411. 2. Link J. After transdermal fentanyl: Acute toxic delirium or central anticholinergic syndrome. Anesthesiology. 1996; 85:436-437. 3. Furbee B, Wermuth M. Life-threatening plant poisoning. Crit Care Clin. 1997; 13:849-888. 4. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:273-274. 5. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:301. ITEM 24 The central nervous system manifestations of central anticholinergic syndrome are attributed to competitive blockade of muscarinic acetylcholine receptors located in the brain. Because atropine and scopolamine are tertiary amines that easily penetrate the blood-brain barrier, they are more likely to produce central anticholinergic syndrome than glycopyrrolate, a quaternary amine that does not readily pass through the blood-brain barrier. Administration of physostigmine has been advocated as a method of diagnosing central anticholinergic syndrome. Unlike quaternary compounds such as edrophonium, neostigmine, and pyridostigmine, which do not penetrate the blood-brain barrier, physostigmine is a tertiary amine that rapidly passes through the blood-brain barrier. Physostigmine is a specific antidote to the treatment of central anticholinergic syndrome and has been described as having efficacy regardless of the causative agent. Given the duration of action of physostigmine (20 minutes) versus that of scopolamine (4-6 hours) and atropine (2-3 hours), repeat administration may be necessary. REFERENCES 1. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:273-274. 2. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:301. 3. Bhanushali MJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin. 2004; 22:389-411. 4. Link J. After transdermal fentanyl: Acute toxic delirium or central anticholinergic syndrome. Anesthesiology. 1996; 85:436-437. 5. Furbee B, Wermuth M. Life-threatening plant poisoning. Crit Care Clin. 1997; 13:849-888. ITEM 25 The sodium nitroprusside molecule contains five cyanide moieties, which are released when an electron is transferred from oxyhemoglobin. Accordingly, cyanide toxicity is a concern in patients receiving sodium nitroprusside, especially those receiving the drug over a prolonged period. 29 Free cyanide may bind cytochrome oxidase and prevent oxidative phosphorylation thereby decreasing oxygen available to cells and producing tissue anoxia, anaerobic metabolism, and metabolic acidosis (lactic acidosis). Plasma lactate concentrations greater than 10 mmol/L tend to correlate with blood cyanide concentrations greater than 40 mmol/L. Concerns about cyanide toxicity tend to limit the duration of use. Monitoring for thiocyanate levels, a marker for cyanide toxicity, is recommended when nitroprusside has been administered o at greater than 4 mcg · kg-1 · min-1 for more than 24 hours o for more than three days patients have o renal insufficiency o liver dysfunction Because oxygen consumption is decrased by the impairment of the cytochrome system, mixed venous oxygen saturation increases with cyanide toxicity. Tachyphylaxis (decreased responsiveness to a constant dose of nitroprusside) is an indication of cyanide toxicity. Dysfunction of the central nervous system (confusion, mental status changes, seizures) is indicative of decreased cerebral cellular oxygenation precipitated by the effects of cyanide on the cytochrome system. REFERENCES 1. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:355-361. 2. Friederich JA, Butterworth JF 4th. Sodium nitroprusside: Twenty years and counting. Anesth Analg. 1995; 81:152-162. ITEM 26 The majority of an administered morphine dose is conjugated to glucuronides. This metabolism occurs in extrahepatic sites, primarily the kidneys, as well as in the liver. Although only 5%-10% of an administered dose is metabolized to morphine-6-glucuronide, this compound has respiratory depressant effects comparable to those of the parent compound and may contribute to long-term analgesia. A small amount of morphine is demethylated to normorphine. Essentially 75%-85% of an administered dose of morphine is metabolized to morphine-3-glucuronide, which has no analgesic activity. Since renal excretion is the primary route of elimination for the morphine metabolites, renal failure may be associated with prolonged ventilatory depression due to the impaired elimination of morphine-6glucuronide. Renal excretion of the parent compound accounts for only 1%-2% of an administered dose. Presumably because extrahepatic sites contribute strongly to morphine clearance, cirrhosis is not associated with a significant decrease in morphine metabolism. REFERENCES 1. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:95. 30 2. Romberg R, Olofsen E, Sarton E, et al. Pharacodynamic effect of morphine-6-glucuronide versus morphine on hypoxic and hypercapnic breathing in healthy volunteers. Anesthesiology. 2003; 99:788-798. 3. Kurella M, Bennett WM, Chertow GM. Analgesia in patients with ESRD: A review of available evidence. Am J Kidney Dis. 2003; 42:217-228. ITEM 27 In general most drugs that alter the response to nondepolarizing neuromuscular blocking drugs are associated with prolongation of the blockade. Antibiotics are the most widely discussed drugs that produce potentiation of neuromuscular blockade. Aminoglycosides and clindamycin potentiate neuromuscular blockade through two mechanisms— decreased release of acetylcholine from nerve terminals and decreased sensitivity of the receptor to acetylcholine. Like local anesthetics, class IA antidysrhythmic drugs, including quinidine and procainamide, potentiate nondepolarizing neuromuscular blocking drugs. This occurs as a result of decreased release of acetylcholine from nerve terminals. Calcium channel blocking drugs, including verapamil, potentiate nondepolarizing neuromuscular blocking drugs by deceasing calcium conductance. Calcium channel blocking drugs may also potentiate neuromuscular blockade produced by depolarizing agents. Chronic anticonvulsant therapy with carbamazepine or phenytoin is associated with resistance to most nondepolarizing neuromuscular blocking drugs. (There is apparently no effect on mivacurium or atracurium but resistance to cisatracurium, rocuronium, vecuronium, and pancuronium has been reported.) In adults this resistance is due to both pharmacokinetic (increased clearance of the drugs) and pharmacodynamic (higher plasma concentrations of the drugs are necessary to produce a specific degree of neuromuscular blockade) mechanisms. In children, however, resistance appears to be related solely to increased clearance. REFERENCES 1. Kaye D. Current use for old antibacterial agents: Polymyxins, rifampin, and aminoglycosides. Infect Dis Clin North Am. 2004; 18:669-689. 2. Richard A, Girard F, Girard DC, et al. Cisatracurium-induced neuromuscular blockade is affected by chronic phenytoin or carbamazepine treatment in neurosurgical patients. Anesth Analg. 2005; 100:538-544. 3. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:225-226. ITEM 28 The American Society for Testing and Materials (ASTM) requires that certain markings be on the outside of all tracheal tubes (Figure 1). These requirements include a radiopaque marker at least at the distal (patient) end of the tube length markings in centimeters measured from the distal (patient) end of the tube internal diameter in millimeters 31 designation of the manufacturer/supplier indication of the intended use (“oral,” “nasal,” or “oral/nasal”) If the tube is a single-use device, it must also bear an indication such as “Single use only” or “Do not reuse.”ASTM standards require that the external diameter (in millimeters) be indicated on the outside of all tracheal tubes with an internal diameter of 6 mm or larger; the standards do not require that the external diameter be listed on tracheal tubes with an internal diameter smaller than 6 mm. Figure 1. Tracheal tube labels. ASTM requirements for tracheal tubes include manufacturer designation (A); internal diameter in millimeters (B); external diameter of the tube (C); if appropriate, an indication that the device is intended for a single use (D); designation of intended use (E); some indication that the material of the tube has passed tissue toxicity testing (F); and length markings in centimeters from the distal end of the tube (G). The radiopaque marker at the distal end of the tube is not visible to the naked eye. (Ryan E. Bowe, photographer) ASTM standards do not require that the tube have a label identifying the material from which it is manufactured, but there must be some indication that the material has passed tissue toxicity testing. This requirement may be fulfilled by labeling the tube with “IT” (for “implant tested”), “F-29” (for Committee F29 on Anesthetic and Respiratory Equipment of the ASTM), or “Z-79” (for Committee Z-79 on Anesthesia Equipment of the American National Standards Institute). REFERENCES 1. Dunn PF, Goulet RL. Endotracheal tubes and airway appliances. Int Anesthesiol Clin. 2000; 38:65-94. 2. Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 4th ed. Baltimore: Williams & Wilkins; 1999:576. 32 ITEM 30 Blood flow in utero is classically described as representing two circulations in parallel. At birth, two primary changes—increase in systemic vascular resistance and decrease in pulmonary vascular resistance—are responsible for converting the circulatory pattern to essentially normal adult circulation. Clamping the umbilical cord causes an increase in systemic vascular resistance as a result of removing the low-resistance placenta from the systemic circulation. The other important change is a decrease in pulmonary vascular resistance associated with the first breath. The ductus arteriosus remains functionally patent for at least 15-20 hours following birth. Some healthy term neonates manifest a patent ductus arteriosus for several days after birth. The foramen ovale is functionally a flap valve that opens when right atrial pressure exceeds left atrial pressure. When open, blood is shunted right-to-left through the foramen. Increases in left atrial pressure result in closure of the foramen ovale; left-to-right shunting does not occur through the foramen ovale. REFERENCES 1. Hong YM, Choi JY. Pulmonary venous flow from fetal to neonatal period. Early Hum Dev. 2000; 57:95-103. 2. Alenick DS, Holzman IR, Ritter SB. The neonatal transitional circulation: A combined noninvasive assessment. Echocardiography. 1992; 9:29-37. 3. Clarke WR. The transitional circulation: Physiology and anesthetic implications. J Clin Anesth. 1990; 2:192-211. 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2007. ITEM 31 Treatment of supraventricular tachycardia should follow the tachycardia algorithm of the updated (2005) Pediatric Advanced Life Support (PALS) guidelines. The 2005 PALS tachycardia algorithm suggests that if no delay occurs a vagal maneuver may be the initial intervention in the treatment of supraventricular tachycardia. Synchronized cardioversion or intravenous adenosine are the first-line treatments to be used either as initial management or if a vagal maneuver has not been effective. Synchronized cardioversion can be immediately performed without the presence of intravenous access. If the child is hemodynamically stable, consideration should be given to sedating the child prior to synchronized cardioversion. In the presence of hemodynamic instability, lengthy delays before cardioversion should not occur. If supraventricular tachycardia is still present after cardioversion and administration of appropriate doses of intravenous adenosine, consultation with a pediatric cardiologist is advised. Additional treatment options for supraventricular tachycardia include the administration of 5 mg/kg admiodarone given intravenously (IV) over 20-60 minutes or 15 mg/kg procainamide given IV over 30-60 minutes. Amiodarone and procainamide should not be administered together (see Figure 1). Defibrillation is not an appropriate treatment option for supraventricular tachycardia. 33 Figure 1. The Pediatric Advanced Life Support (PALS) Tachycardia Algorithm. Obtained from the 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care; available online at: http://circ.ahajournals.org/cgi/content/full/112/24_suppl/IV167/FIG3 34 REFERENCES 1. American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care; Part 12: Pediatric Advanced Life Support. Circulation. 2005; 112:IV-167-IV-187. Available online at http://circ.ahajournals.org/cgi/content/full/112/24_suppl/IV-167/FIG3. Accessed February 2007. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2839, 2930. ITEM 32 A patient is scheduled for external fixator placement to the right leg after being involved in a motor vehicle accident. The patient is intubated, ventilated, and has a chest tube in place. On arrival in the operating room, vital signs include a blood pressure of 78/52 mm Hg and oxygen saturation 80% with FIO2 = 1.0. Findings on chest auscultation include wheezing and decreased breath sounds over the left axilla. The patient’s chest radiograph is shown in Figure 1. The differential diagnosis for hypoxemia, wheezing, and decreased breath sounds includes bronchospasm, mainstem intubation, presence of a foreign body, pneumothorax, and occluded tracheal tube. In reviewing the chest radiograph (Figure 1), a left-sided pneumothorax is present; the tracheal tube appears to be properly positioned. The treatment of choice is placement of a thoracostomy tube on the left. Figure 1. A chest radiograph depicting a left-sided pneumothorax with the tracheal tube in an appropriate position. 35 Pneumothorax is not uncommon among patients with traumatic injuries. Patients with a history of traumatic injuries should have a preoperative chest radiograph to exclude cardiothoracic injuries in addition to inquiring about shortness of breath or chest pain. Chest tube placement prior to positive pressure ventilation should be considered for all patients with a pneumothorax. A pneumothorax develops when gas collects within the pleural space, resulting in lung collapse. If the air in the pleural space cannot be released due to a one-way valve effect, a tension pneumothorax is present. Atelectasis and intrapulmonary shunting result from compression of the lung. Compression of mediastinal structures such as the vena cava may produce hypotension. Treatment of a tension pneumothorax includes prompt identification and needle decompression or tube thoracostomy. After placing a needle or intravenous catheter in the midclavicular line of the upper border of the second rib, a sudden rush of air should be detectable. Chest tube placement should then follow. Administering albuterol or repositioning the tracheal tube are not appropriate treatments for a pneumothorax. Bronchoscopy would be appropriate to check for airway obstruction (by foreign body, mucous plug, etc.) but is not the appropriate management for a pneumothorax. REFERENCES 1. de Lassence A, Timsit JF, Tafflet M, et al. Pneumothorax in the intensive care unit: Incidence, risk factors, and outcome. Anesthesiology. 2006; 104:5-13. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:1902, 2713. ITEM 33 Based on various reports, 1%-2% of parturients require nonobstetric surgery during their pregnancies. When a surgical procedure can be performed via a laparoscopic approach, many of the advantages over open surgery that exist in nonpregnant patients (faster recovery, less postoperative pain) are also significant advantages in parturients. When laparoscopic surgery was first introduced, concerns were expressed about the effects of this technique on the parturient and fetus. However, over 500 successful laparoscopic surgeries in pregnant women have now been reported in the medical literature. As a result, laparoscopy in pregnancy is considered safe and is not contraindicated. Some unique issues must be considered when performing laparoscopy in the parturient. To prevent trauma to the fetus or gravid uterus, an open trochar technique should be utilized. The hemodynamic and respiratory effects of CO2 insufflation and increased intraabdominal pressure raised the greatest concerns when laparoscopy was first performed in pregnant women. Study results as well as the experience of practitioners performing laparoscopy in pregnant patients indicate that maintaining a relatively low insufflation pressure of 12-15 mm Hg avoids the adverse effects associated with insufflation. The need for intraoperative arterial blood gas monitoring is controversial. Based on the reported development of fetal acidosis during CO2 insufflation in pregnant sheep models, the Society of American Gastrointestinal Endoscopic Surgeons published guidelines that recommend blood gas monitoring in parturients undergoing laparoscopic surgery. However, in a study of healthy parturients undergoing laparoscopic surgery using an insufflation pressure of 15 mm Hg, the PaCO2-ETCO2 gradient was small. By adjusting minute ventilation to maintain an ETCO2 of 32 mm Hg, the authors found that normal maternal PaCO2 and pH could be achieved throughout surgery. Therefore, many anesthesiologists believe arterial blood gas monitoring is not necessary in healthy pregnant women. While laparoscopic surgery offers advantages over laparotomy for the mother, including a significantly shorter hospitalization, the effects of a laparoscopic approach compared to open surgery on fetal outcome 36 must also be considered. The risk of delivering a premature, low birth weight infant is increased in pregnant patients undergoing either laparoscopy or laparotomy compared to parturients who do not undergo surgery. However, the risks of fetal loss, preterm delivery, and congenital malformations do not differ between the two surgical approaches. Fetal heart rate monitoring could be beneficial in assessing the physiologic effects of laparoscopy on the fetus. However, continuous intraoperative fetal heart rate monitoring during this surgical approach is not easily performed and can only be achieved with the use of a transvaginal ultrasound probe. Therefore, fetal heart rate monitoring is most commonly used only during the preoperative and postoperative periods in parturients undergoing laparoscopic surgery. REFERENCES 1. Chestnut DH. Obstetric Anesthesia: Principles and Practice. 3rd ed. Philadelphia: Elsevier Mosby; 2004:266-267. 2. O’Rourke N, Bhavani-Shankar K. Laparoscopic surgery during pregnancy. Curr Opin Anaesthesiol. 2006; 19:254-259. 3. Bhavani-Shankar K, Steinbrook RA, Brooks DC, et al. Arterial to end-tidal carbon dioxide pressure difference during laparoscopic surgery in pregnancy. Anesthesiology. 2000; 93:370-373. ITEM 34 Patients in whom an excessive amount of growth hormone is present prior to fusion of the epiphyseal growth plates develop gigantism. In patients after puberty, excessive secretion of growth hormone causes acromegaly. Acromegaly occurs in 3-4 people per million of the population. The features of acromegaly include a general overgrowth of skeletal, soft, and connective tissues, resulting in coarse facial features and enlarged hands and feet. Of concern to anesthesiologists is the alteration of airway anatomy. The tongue and epiglottis may be enlarged and the mandible may show excessive growth, resulting in increased distance from the lips to the vocal cords. The vocal cords may be enlarged with narrowing of the glottis and the subglottic area. Nasal turbinate enlargement may occur, as may paralysis of the recurrent laryngeal nerves. These anatomical changes may predispose to the development of sleep apnea, to difficulties in attaining a good mask seal, and to difficulties in placing a tracheal tube. Consideration should be given to the use of an awake fiberoptic technique to secure the airway. Systemic features of acromegaly include hypertension and hyperglycemia. Congestive heart failure may also occur secondary to a cardiomyopathy in which diastolic dysfunction and dysrhythmias are prominent. Ventilation/perfusion mismatch, peripheral neuropathy, arthritis, and inadequate ulnar collateral flow may also occur. Acromegaly is associated with overt diabetes mellitus in 10%-15% of cases and with insulin resistance in 50% of cases. A basophilic adenoma of the adenohypophysis (anterior pituitary gland) that secretes growth hormone is the usual cause of acromegaly. Prolactin may be co-secreted with growth hormone and is increased in 30% of patients with acromegaly. The secretion of other anterior pituitary hormones (especially the gonadotrophins follicle-stimulating hormone and luteinizing hormone) is usually suppressed. Adrenocorticotrophic hormone (ACTH) is secreted by the anterior pituitary as part of the hypothalamicpituitary-adrenal axis. Excess secretion causes Cushing disease, although the levels may be reduced if a primary adrenal source secretes excess cortisol or if excessive exogenous cortisol is administered (Cushing syndrome). 37 REFERENCES 1. Nemergut EC, Dumont AS, Barry UT, et al. Perioperative management of patients undergoing transsphenoidal pituitary surgery. Anesth Analg. 2005; 101:1170-1181. 2. Schmitt H, Buchfelder M, Radespiel-Troger M, et al. Difficult intubation in acromegalic patients: Incidence and predictability. Anesthesiology. 2000; 93:110-114. 3. Seidman PA, Kofke WA, Policaro R, et al. Anaesthetic complications of acromegaly. Br J Anaesth. 2000; 84:179-182. 4. Faust RJ. Anesthesiology Review. 3rd ed. New York: Churchill Livingstone; 2002:379-380. 5. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:10511052. ITEM 35 Scenario: In a recently published paper the authors discuss the effectiveness of a new drug (compound Q) used to prevent the development of cognitive dysfunction after cardiopulmonary bypass (CPB). In the results section, the authors state that “the relative risk of the development of cognitive impairment in the placebo group compared with the group administered compound Q was 1.3 (95% confidence interval 0.7 to 1.9).” The relative risk (also known as the risk ratio) is a ratio of two proportions. The relative risk of developing a disease is expressed as the ratio of the risk (incidence) in individuals exposed to potential risk factor (or a potential protective factor) to the risk in unexposed individuals: Risk of disease in group 1 / Risk of disease in group 2 = Relative risk The relative risk should be reported with its confidence intervals, usually the 95% confidence intervals; that is, the authors are 95% confident that the interval between the upper and lower confidence levels contains the true population relative risk. If the value 1 is in the interval (as in the question), the difference is not statistically significant with the P value less than 0.05 and the difference observed is likely due to chance. In most circumstances if the relative risk is greater than 1 (and the confidence interval does not include 1), then the risk of disease in group 1 is greater than the risk of the disease in group 2. For example, the relative risk of coronary artery disease in smokers (group 1) compared with nonsmokers (group 2) is 4, indicating that smoking is associated with coronary artery disease. In therapeutic trials, the experimental group can be compared to groups receiving no therapy, a placebo, or an established therapy. If group 1 is a treatment group and group 2 is a control group and the relative risk of developing a disease is less than 1 (and the confidence interval does not include 1), then the treatment is effective, which means that it is either an effective treatment (when the comparison is made to an untreated group) or a more effective treatment (when the comparison is made to a different treatment). For example, in the Physicians Health study, the risk of developing a myocardial infarction in those physicians taking aspirin was 0.94%. The risk of myocardial infarction in those taking placebo was 1.71%. The relative risk of myocardial infarction in the aspirin group compared with the placebo group was 0.94/1.71 = 0.55/ Thus, physicians taking aspirin were 0.55 times (55%) as likely to have a myocardial infarction as those taking placebo. To calculate the risk ratio for the control group compared to the treatment group, the inverse of the risk ratio for treatment compared to controls is determined. In 38 this example, the controls were 1/0.55 = 1.82 times as likely to have a myocardial infarction as those taking aspirin. Bias is the result of a systematic error in the design or conduct of a study. This systematic error results from flaws either in the method of selection of study participants or in the procedures for gathering relevant exposure and/or disease information. Consequently, the observed study results will tend to be different from the true results. It is not possible to tell from the information given in the question whether or not the study of compound Q was biased. REFERENCES 1. Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Sudbury, Massachusetts: Jones and Bartlett; 2004:92-98, 125-126. 2. Gordis L. Epidemiology. 3rd ed. Philadelphia: Saunders; 2004:179-181. 3. Riegelman RK. Studying a Study and Testing a Test. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005:58-62. ITEM 36 Venous air embolism (VAE) may occur during placement or removal of a central venous catheter and is a potentially fatal complication. VAE can occur whenever a vein is opened and a pressure gradient exists that favors air entrainment rather than bleeding. Classically, neurosurgical procedures with the patient in the sitting position (where the operative site is above the level of the heart) have been associated with VAE. The amount of air entrained will depend on the pressure gradient from the atmosphere into the circulation, the size of the hole in the vessel wall, and the duration of the entrainment. The signs and symptoms of VAE depend on the amount of air entrained and the rate of entrainment. Massive VAE may create an “air lock” in the right ventricle leading to right ventricular outflow obstruction and cardiovascular collapse. Entrainment of smaller amounts of air leads to an increase in pulmonary vascular resistance because of mechanical obstruction of small pulmonary arteries and arterioles and the release of endogenous vasoconstrictors. An increase in pulmonary vascular resistance will increase right ventricular afterload, increase pulmonary artery pressure, and lead to right ventricular failure. A subsequent decrease in cardiac output will cause hypotension. At the same time as the hemodynamic changes, the patient may become dyspneic and hypoxemic and complain of chest pain. The diagnosis of a large VAE is usually clinical. For smaller VAE echocardiography is the most sensitive diagnostic test. In neurosurgical procedures in the sitting position, continuous use of a precordial Doppler may also alert the clinician to air entrainment. Suggestions for decreasing the risk of VAE when inserting or removing venous catheter include: using the Trendelenburg position (except for femoral catheters) administering a fluid bolus to avoid hypovolemia asking the patient to perform a Valsalva maneuver (especially as the catheter is being removed) placing an occlusive dressing over the site of the catheter’s entry into the skin Deep inspiration should be avoided as it will decrease intrathoracic pressure and increase the risk of entraining air into the venous circulation. Patients on positive pressure ventilation are more likely to entrain air into the circulation. 39 If VAE occurs, the patient should be placed with the left side down (to “trap” air in the right ventricle and avoid dispersal into the pulmonary circulation), the site of entry should be occluded, 100% oxygen should be administered (to increase the rate of resorption of the entrained air), and methods to support cardiopulmonary function should be initiated. If a patient is undergoing general anesthesia, the use of nitrous oxide should be discontinued as it will tend to increase the size of the embolism. Rarely, a paradoxical air embolism will occur in which gas in the venous system gains access to the arterial circulation (eg, through a patent foramen ovale). If a paradoxical embolism causes neurologic sequelae, urgent arrangements for hyperbaric oxygen therapy should be made. REFERENCES 1. Pronovost PJ, Wu AW, Sexton JB. Acute decompression after removing a central line: Practical approaches to increasing safety in the intensive care unit. Ann Intern Med. 2004; 140:1025-1033. 2. Needham DM, Sinopoli DJ, Thompson DA, et al. A system factors analysis of “line, tube, and drain” incidents in the intensive care unit. Crit Care Med. 2005; 33:1701-1707. 3. Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000; 342:476-482. 4. Murray MJ, Coursin DB, Pearl RG, et al. Critical Care Medicine: Perioperative Management. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002:528. ITEM 37 A 55-year-old man in shock is being care for in the intensive care unit. In an effort to determine the cause of the shock, a pulmonary artery catheter is placed. The following data are recorded: Systemic blood pressure Central venous pressure Pulmonary artery pressure Pulmonary artery occlusion pressure Cardiac index Heart rate 80/60 mm Hg 18 mm Hg 32/18 mm Hg 18 mm Hg 1.6 L/min/m2 138 beats/min Pericardial tamponade exists when intrapericardial pressure, not intravascular volume and venous pressure, determines venous return. The ventricle is small and underfilled despite increases in right and left ventricular filling pressures. Clinically, this may manifest as hypotension, pulsus paradoxus (an inspiratory fall of systolic blood pressure greater than 10 mm Hg), and Kussmaul sign (a reversal of the normal pattern of decreasing jugular venous pressure during inspiration). When a pulmonary artery catheter is in situ in a patient with pericardial tamponade, a tendency towards equalization of diastolic pressures is seen. This occurs because the fluid-filled pericardium compresses all the chambers of the heart. In this patient the central venous pressure is equal to the pulmonary artery diastolic pressure and the pulmonary artery occlusion pressure. Pericardial tamponade will also decrease the stroke volume leading to a decrease in the cardiac index. The heart rate will reflexively increase in an effort to maintain cardiac index. Patients may present with tachycardia, hypotension, dyspnea, and orthopnea. If a patient is intubated, ventilated, and sedated after, for example, cardiac surgery, hypotension may be the only clinical sign. A pulmonary embolism would manifest with increased right-sided (central venous and pulmonary artery) pressures. However, underfilling of the left ventricle will occur and, in contrast to the situation in 40 pericardial tamponade, there is no extrinsic compression on the left ventricle, so the pulmonary artery occlusion pressure would be low. Patients with hypovolemic shock, such as after a massive gastrointestinal bleed, will have low right- and left-sided filling pressures, in contrast to the values given for this patient. Anaphylaxis (along with septic and neurogenic shock) is a form of distributive shock, which is typified by a hyperdynamic circulation and a high cardiac index. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:918-919. 2. Fiedler M, Nelson LA. Cardiac tamponade. Int Anesthesiol Clin. 2005; 43(4):33-43. 3. Oh JK, Seward JB, Tajik AJ. The Echo Manual. 2nd ed. Philadelphia: Lippincott-Raven; 1999:181-194. ITEM 38 Clinical scenario: An intubated patient is being transferred from the operating room to the intensive care unit on a different floor of the hospital with a full cylinder of oxygen. During transport, the elevator becomes stuck. The flow setting on the cylinder is on 10 L/min oxygen. A full E cylinder of oxygen contains 660 L of oxygen at 1,900 psig (Table 1). At a rate of 10 L/min, a full cylinder should last approximately 66 minutes. Table 1. Pressure and volume of nonliquefied oxygen gas in tanks from full to empty. Full Tank Half Full Quarter Full Empty 1,900 psig 950 psig 475 psig 0 psig 660 L 330 L 165 L 0L 1. Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 4th ed. Baltimore: Williams & Wilkins; 1999:9-15. 2. Schumacher SD, Brockwell RC, Andrews JJ, et al. Bulk liquid oxygen supply failure. Anesthesiology. 2004; 100:186-189. ITEM 39 Remifentanil is the only ultrashort-acting opioid that has become widely available for use in a variety of clinical settings. It has a predictable offset of opioid drug effect due to its unique pharmacokinetic properties. Rapid metabolism of this drug occurs through hydrolysis of its esterase linkages by blood- and tissue-nonspecific esterases, such as nonspecific cholinesterase, and is independent of elimination by organs. Remifentanil is not affected by plasma cholinesterase deficiency, however. 41 Context-sensitive half-time is the time required for a drug’s plasma concentration to decrease by 50% after an infusion is stopped. Remifentanil is primarily used as an infusion due to the short half-time of three to four minutes and can be combined with propofol infusions for maintenance of total intravenous anesthesia. Its context-sensitive half-time remains short so that even after being infused for 600 minutes at high doses, patients can emerge from anesthesia in only six minutes when remifentanil is combined with propofol, after discontinuing both infusions. This is unlike fentanyl or sufentanil, which both have longer context-sensitive half-times, and patients receiving these drugs as infusions demonstrate progressively longer emergence times as the duration of the infusions increase. As the clearance for remifentanil is higher than that of normal hepatic blood flow, there is no significant hepatic extraction or redistribution. Metabolism is extrahepatic, and remifentanil’s metabolites are essentially inactive at the mu receptor. There is also no significant renal elimination of remifentanil, thus neither liver failure nor renal failure has an effect on the pharmacokinetics of this drug. REFERENCES 1. Beers R, Camporesi E. Remifentanil update: Clinical science and utility. CNS Drugs. 2004; 18:1085-1104. 2. Castanelli DJ, Splinter WM, Clavel NA. Remifentanil decreases sevoflurane requirements in children. Can J Anaesth. 2005; 52:1064-1070. 3. Kee WD, Khaw KS, Ma KC, et al. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: A randomized, double-blind, controlled trial. Anesthesiology. 2006; 104:14-20. 4. Steinlechner B, Koinig H, Grubhofer G, et al. Postoperative analgesia with remifentanil in patients undergoing cardiac surgery. Anesth Analg. 2005; 100:1230-1235. 5. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:400415, 446-455. ITEM 40 The interscalene block is frequently used as regional anesthesia to provide anesthesia and analgesia for surgery on the shoulder and upper humerus. It is also ideal for reduction of a separated shoulder. With an interscalene block, the brachial plexus is blocked at the level of the primary trunks. Typically the superior (C5-6) and middle (C7) trunks are effectively blocked, leaving the inferior (C8-T1) trunk and resultant branches spared. Consequently, this block is generally not appropriate for surgery on the hand, particularly the medial hand and forearm. The interscalene block is often performed at the level of the C6 vertebral body between the anterior and middle scalene muscle. Due to its proximity, the phrenic nerve is frequently blocked in this approach leading to ipsilateral diaphragm paralysis. Consequently, this block should not be done in patients with severe pulmonary disease or those who are dependent on accessory respiratory muscles while breathing at rest. The infraclavicular block provides analgesia and anesthesia to the arm, elbow, and hand. It blocks the brachial plexus at the level of the cords. The axillary blocks provides blockade of the terminal nerves and is effective for surgery on the hand and the arm distal to the elbow. The cervical plexus is derived from C1-4 spinal nerves, and blockade provides analgesia and anesthesia to the anterolateral neck and the anteand retroauricular regions. Indications for its use include carotid endarterectomy and neck surgery. 42 REFERENCES 1. Brown DL. Atlas of Regional Anesthesia. 2nd ed. Philadelphia: WB Saunders; 1999:25-19. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:16881689. 3. Evans H, Steele SM, Nielson KC, et al. Peripheral nerve blocks and continuous catheter techniques. Anesthesiol Clin North America. 2005; 23:141-162. ITEM 41 Phantom limb pain is one of three distinct elements occurring after amputation: phantom sensations (mostly normal sensations, except for telescoping where the lost limb feels presents and much smaller), phantom limb pain, and stump pain. Only the last two require pain therapy and will be considered here. Phantom limb pain occurs in 60%-80% of patients within the first week of limb loss but rarely can occur several years later. While the initial pain tends to last as long as two years, a survey of 526 veterans reported eventual fading in a slim majority; phantom limb pain vanished in 16% lessened markedly in 37%, remained the same in 44%, and increased in 3%. Phantom limb pain is neuropathic-type pain in the absent and distal part of the limb. It is an intermittent, rarely constant pain of a stabbing, pricking, boring, squeezing, shooting, throbbing or burning nature that is fairly resistant to all forms of therapy. After proper healing, persistent pain in the distal stump is considered stump pain and may exist long term in 25%-60% of these patients. Stump pain is described as pressing, burning, throbbing, stabbing, and/or an electric current sensation (neuroma) usually in intermittent painful attacks (nerve storms), plus spontaneous jerks of the stump. Examination of the stump will often reveal causes of stump pain such as infection, bone spurs, neuromas, etc. Noninvasive medical approaches to stump pain and phantom limb pain have included treatment with membrane-stabilizing drugs (ie, sodium channel blockers), antidepressants, N-methyl-D-aspartate (NMDA) antagonists, calcitonin, gabapentin, clonidine, and opioids. Results have been mixed and randomized controlled trials sparse. Tramadol and amitriptyline are examples of drugs whose efficacy for this type of neuropathic pain has been confirmed by randomized controlled trials. A trial of opioid therapy should always be performed for phantom limb pain not responding to nonopioid medical therapy. Invasive surgical treatments have demonstrated no lasting effect; even spinal cord stimulation has provided less than optimal results. The search continues for better noninvasive medical solutions. REFERENCES 1. Wilder-Smith CH, Hill LT, Laurent S. Postamputation pain and sensory changes in treatmentnaïve patients: Characteristics and responses to treatment with tramadol, amitriptyline, and placebo. Anesthesiology. 2005; 103:619-628. 2. Melzack R, Wall PD. Handbook of Pain Management. New York: Churchill Livingstone; 2003:247-257. ITEM 42 Pheochromocytomas may secrete a variety of catecholamines and other vasoactive substances. Norepinephrine secretion produces sustained hypertension and bradycardia. Epinephrine secretion 43 typically produces episodic sweating, tremor, headache, hypertension, and tachycardia. Dopamine secretion is usually associated with nausea and vomiting. Hypertension and catecholamine excess can result in cardiomyopathy. Preoperative alpha-blockade is typically induced with the long-acting, nonselective alpha-antagonist phenoxybenzamine. During induction of this alpha-blockade, intravascular volume resuscitation is required as these patients are characteristically hypovolemic. Beta-blockade is typically indicated for tumors that secrete epinephrine. Beta-blockade in the absence of adequate alpha-blockade can precipitate a severe hypertensive crisis—circulating catecholamines trigger severe vasoconstriction due to alphaagonism without opposition from vasodilating beta2-agonism. The following criteria have been proposed to indicate adequate preoperative preparation of the patient scheduled for pheochromocytoma resection: all blood pressure readings should be below 160/90 mm Hg in the 24 hours prior to surgery the patient should be orthostatic but blood pressure when standing should be greater than 80/45 mm Hg the electrocardiogram should be free of ST-T changes for at least one week the patient should have no more than one premature ventricular contraction every five minutes Adverse perioperative outcomes have been demonstrated in patients undergoing pheochromocytoma resection when these preoperative criteria were not met. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1039-1060. 2. Kinney MA, Narr BJ, Warner MA. Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. 2002; 16:359-369. 3. Bogdonoff DL. Pheochromocytoma: Specialist cases that all must be prepared to treat? J Cardiothorac Vasc Anesth. 2002; 16:267-269. ITEM 43 Vasopressin is a nonapeptide that is synthesized in the hypothalamus and released from the posterior pituitary. The important triggers for vasopressin release include increased plasma osmolality, decreased arterial pressure, and decreased blood volume. There are three subtypes of vasopressin receptors: V1 receptors. V1 stimulation results in vasoconstriction. These receptors are located in the central nervous system, vascular smooth muscle cells, liver, and platelets. V2 receptors. V2 stimulation causes water retention. These receptors are found primarily in the collecting ducts cells of kidneys. V3 receptors. V3 stimulation results in corticotrophin secretion. These receptors are located primarily in the anterior pituitary gland. High-dose vasopressin therapy may produce myocardial ischemia. Exogenous vasopressin reduces uterine blood flow significantly in both pregnant and nonpregnant states. No data exist on the use of vasopressin to manage hypotension during obstetric anesthesia. Vasopressin currently cannot be recommended as a suitable vasopressor during anesthesia for labor and delivery. 44 There is a relative vasopressin deficiency in septic shock. Exogenous vasopressin therapy in patients with septic shock increases systemic vascular resistance and arterial blood pressure. Furthermore, creatinine clearance and urine production are also improved. Additional studies are required to document whether these favorable effects due to vasopressin therapy reduce mortality from sepsis. There is no evidence that vasopressin therapy is associated with increased mortality in sepsis. Vasodilatory shock refractory to catecholamine therapy may occur in association with septic shock, anaphylaxis, severe hemorrhage, cardiopulmonary bypass, and pheochromocytoma resection. In all these scenarios, exogenous vasopressin therapy (by bolus and/or infusion) has been shown to reduce vasopressor requirements and restore systemic vascular resistance to arterial blood pressure. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:737. 2. Treschan TA, Peters J. The vasopressin system: Physiology and clinical strategies. Anesthesiology. 2006; 105:599-612. 3. Sharma RM, Setlur R. Vasopressin in hemorrhagic shock. Anesth Analg. 2005; 101:833-834. ITEM 44 Postdural puncture headache (PDPH) is generally defined as a headache developing within days after dural puncture and resolving spontaneously within one week or within 48 hours after effective treatment of the spinal fluid leak (usually by epidural blood patch). The headache worsens within 15 minutes after sitting or standing and improves within 15 minutes after lying down. Some patients experience additional signs and symptoms including neck stiffness, tinnitus, hypacusia (decreased hearing), photophobia, diplopia, or nausea. Typically the headache is located in the frontal or occipital areas, or both. The incidence of PDPH in adults is inversely associated with age. The greatest factor affecting the incidence of PDPH is the choice of needle and technique. Using cutting needles such as the Quincke it is helpful to have the direction of the bevel parallel to the longitudinal axis of the spine. Smaller needles have been shown to reduce the incidence. Additionally, the incidence is reduced with the use of a pencil point needle. There is no evidence that the addition of vasoconstrictors increases the severity of the PDPH. At one time it was thought that maintaining a supine position after dural puncture would reduce the incidence of PDPH. This has been determined not to be true and bed rest is not longer recommended unless symptoms occur. REFERENCES 1. Gaiser R. Postdural puncture headache. Curr Opin Anaesthesiol. 2006; 19:249-253. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2328. ITEM 45 Treatments for postdural puncture headache (PDPH) range from conservative to invasive. Typical conservative measures include bed rest, analgesics, caffeine, and hydration. Presumably, but awaiting proof, the analgesic effects of caffeine are related to its cerebral vasoconstriction properties. While headache relief with oral caffeine has been demonstrated, the pain has a high recurrence rate following this form of therapy. Sumatriptan succinate (Imitrex), a serotonin receptor agonist used for migraine treatment, has been effective in treatment of PDPH. Another migraine medication, methylergonovine 45 maleate (Methergine), was also recently studied in 25 patients who developed PDPH following spinal anesthesia for cesarean delivery. Twenty-four of these patients had improvement within one day and only one required an epidural blood patch (EBP). EBP remains the gold standard to which all other treatments for PDPH are compared. Pain relief is often immediate following an EBP. In fact, it is this immediate response that casts doubt on the theory that the benefit is primarily from plugging the dural leak. If so, it would be expected that the loss of cerebrospinal fluid (CFS) would take much longer to replace. It is postulated that the EBP also works by compression of the thecal sac resulting in increased subarachnoid pressure that forces CSF cephalad. The timing of the EBP continues to be debated. There are studies that suggest that early placement of an EBP leads to increased failure. Recently the amount of time a patient was bedridden and the efficacy of the EBP was studied by Vilming et al. They concluded that the epidural blood patch should be performed after an observation and conservative treatment period of 24 hours. This time delay increases the rate of success of the EBP while reducing patient suffering. Many physicians have their patients maintain a decubitus or supine position for 30 minutes after placement of an EBP. There is some evidence that longer delays before ambulation may make the EBP more successful. REFERENCES 1. Carp H, Singh PH, Vadhera R, et al. Effects of the serotonin receptor agonist sumatriptan on postdural puncture headache: Report of six cases. Anesth Analg. 1994; 79:180-182. 2. Hakim S, Khan RM, Maroof M, et al. Methylergonovine maleate (Methergine ) relieves postdural puncture headache in obstetric patients. Acta Obstet Gynecol Scand. 2005; 84:100. 3. Vilming ST, Kloster R, Sandvik L. When should an epidural blood patch be performed in postlumbar puncture headache? A theoretical approach based on a cohort of 79 patients. Cephalalgia. 2005; 25:523-527. 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2328. ITEM 46 Pain after laparoscopic cholecystectomy is important: it prolongs hospital stay in up to 40% of patients. The overall postoperative pain results from three separate nociceptive mechanisms: somatic (incisional), visceral (deep intraabdominal pain), and/or referred visceral (shoulder pain). The pain is most intense for the first 48 postoperative hours, typically declining to low levels within four days. In up to 13% of patients, pain may remain severe for up to a week postoperatively. Chronic pain develops in up to 12% of patients after laparoscopic cholecystectomy, and in up to 50% of these chronic cases there is no demonstrable pathology. These chronic pain patients are also significantly more likely to experience intense acute pain after laparoscopic cholecystectomy. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:22852306. 2. Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: A critical assessment of the evidence. Anesthesiology. 2006; 104:835-846. 46 3. Bisgaard T, Rosenberg J, Kehlet H. From acute to chronic pain after laparoscopic cholecystectomy: A prospective follow-up analysis. Scand J Gastroenterol. 2005; 40:13-1364. ITEM 47 Clinical scenario: Shortly after undergoing a C7 transforaminal epidural injection of steroid and bupivacaine under fluoroscopic guidance for treatment of a cervical radiculopathy a previously healthy 54-year-old man requires admission to the intensive care unit. A brainstem infarction is documented by magnetic resonance imaging and the patient dies within 24 hours. Pain in a cervical nerve root distribution is caused by irritation of that spinal nerve root and is thought to occur in approximately one person per 1,000 per year. The most likely cause is a herniation or neuroforaminal stenosis. Typically the prognosis is favorable with the vast majority improving with time and conservative therapy. Nonetheless, a subset of patients do not respond to conservative therapy and seek nonsurgical alternative options. One of these options that have gained popularity is the transforaminal injection of local anesthetic and steroids. The rationale for transforaminal cervical epidural steroid injections is to deliver the steroid directly onto the nerve root to reduce inflammation. The transforaminal approach has recently gained popularity over the traditional translaminar approach as it directs the needle right to the site of the presumed pathology, thereby delivering the medication closer to the irritated nerve (Figure 1). Figure 1. Illustration of an axial view of the cervical intervertebral foramen and adjacent structures at the level of C6 with a needle inserted parallel to the axis of the foramen along its posterior wall. Note the proximity of adjacent structures: C6 = vertebral body of C6; CA = common carotid artery; IJV = internal jugular vein; sap = superior articular process of C5-C6 zygapophysial joint; ScA = anterior scalene muscle; ScM = middle scalene muscle; VA = vertebral artery. Used with permission, from Rathmell JP, Aprill C, Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004; 100:1595-1600. The procedure is typically performed under fluoroscopic or computer tomography guidance by experienced pain physicians. Several observational studies suggest cervical transforaminal injection of steroids results in significant reduction in pain and increase in function, but controlled studies are needed to determine efficacy. Furthermore, there are no studies comparing transforaminal to translaminar approaches. 47 There have been several recent reports of complications associated with transforaminal cervical steroid injection, with some being catastrophic. Specifically, presumed injection of particulate steroids into the radicular arteries has led to spinal cord injury and vertebral artery injection has resulted in brainstem injury. Direct injury to the vertebral artery with subsequent dissection and brainstem injury resulting in severe neurologic damage or death has been reported. These complications have led some opponents to call for abandonment of the procedure due to the lack of controlled studies demonstrating efficacy. Proponents advocate that it is an efficacious procedure but that additional measures such as the use of digital subtraction fluoroscopy should be used to detect inadvertent vascular injection. With appropriate supportive care, allergic reaction from the local anesthetic would not likely result in death. Similarly, intrathecal injection of local anesthetic and steroid would not be expected to result in death. While sepsis and death have been reported from epidural injection of contaminated supplies of steroid, it has been a delayed reaction (occurring over a period of days). REFERENCES 1. Baker R, Dreyfuss P, Mercer S, et al. Cervical transforaminal injection of corticosteroids into a radicular artery: A possible mechanism for spinal cord injury. Pain. 2003; 103:211-215. 2. Rathmell JP, Aprill C, Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004; 100:1595-1600. 3. Rathmell JP, Benzon HT. Transforaminal injection of steroids: Should we continue? Reg Anesth Pain Med. 2004; 29:397-399. 4. Rozin L, Rozin R, Koehler SA, et al. Death during transforaminal epidural steroid nerve root block (C7) due to perforation of the left vertebral artery. Am J Forensic Med Pathol. 2003; 24:351-355. 5. Rathmell JP, Aprill C, Bogduk N. Cervical transforaminal injection of steroids. Anesthesiology. 2004; 100:1595-1600. 6. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:27732774. ITEM 48 Clinical scenario: An otherwise healthy 35-year-old male is scheduled for a 12-hour reconstructive surgical procedure. Following inadvertent rapid administration of 250 mcg of sufentanil, the patient becomes apneic and positive pressure ventilation with a bag and mask is difficult. Difficult ventilation has been reported after administration of all opioids. Although the exact mechanism is not clear, the most likely cause is upper airway obstruction. Proposed mechanisms include primary vocal cord closure, supraglottic obstruction, and central mu receptor activation. A direct muscular action (chest wall rigidity) is not thought to be a part of the mechanism of difficult ventilation from high-dose opioid administration. This complication is more likely to occur when the dose is high and administration is rapid. Difficult ventilation following opioid administration is effectively treated with neuromuscular blocking agents. Midazolam and diazepam also have been used in the treatment of opioid-related difficult ventilation. If clinically indicated, opioid antagonists such as naloxone may also be considered in the treatment of opioid-related difficult ventilation. 48 Albuterol, ketamine, and volatile anesthetic agents are not the most appropriate treatment options for a patient with difficult ventilation from high-dose opioid administration. REFERENCES 1. Fahnestich H, Steffan J, Kau N, et al. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000; 28:836-839. 2. Abrams JT, Horrow JC, Bennett JA, al. Upper airway closure: A primary source of difficult ventilation with sufentanil induction of anesthesia. Anesth Analg. 1996; 83:629-632. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:390391. ITEM 49 Dexmedetomidine is a potent alpha2 agonist with about 1,600-fold greater selectivity for alpha2 than alpha1 receptors. It has approximately seven times more affinity for alpha2 receptors than clonidine does and a shorter half-life. Dexmedetomidine is rapidly redistributed and extensively metabolized by the liver and has been shown to be an effective anxiolytic. It provides sedation, analgesia, anxiolysis, hypnosis, and sympatholysis. Properties of dexmedetomidine include its ability to: decrease minimum alveolar concentration of volatile agents provide hemodynamic stability act as an antisialagogue provide potent analgesia effectively treat shivering effectively blunt the response of laryngoscopy In animals dexmedetomidine was able to attenuate the rigidity caused by opioids. Loading of the drug can result in paradoxical hypertension, which then usually decreases when central blockade is established. Dexmedetomidine is currently only approved for short-term sedation (< 24 hours). The role of this drug may expand as an adjunct to other anesthetics, acting to reduce the opioid and hypnotic requirements. It may also be useful in decreasing sympathetic discharge in patients at risk for myocardial ischemia. REFERENCES 1. Andrea P, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol. 2005; 18:412418. 2. Gertler R, Brown HC, Mitchell DH, al. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001; 14:13-21. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:315-316. 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:355359. ITEM 50 Pulse oximetry is a simple, inexpensive way to measure oxygen saturation (SPO2). It uses the technology of plethysmography to trace the pulsatile volume changes and spectrophotometry to measure oxygen 49 saturation. The pulse oximeter probe contains two light-emitting diodes (LEDs) for transmitting and a photodetector (transducer) for sensing. These are placed on opposite sides of the finger and pulsatile signals across tissue are measured at two discrete wavelengths. These signals are then normalized by using the absorption caused by everything except the pulsatile blood. A ratio between the two normalized signals is computed and this ratio is then related to oxygen saturation empirically using an algorithm. The majority of pulse oximeters base these calculations on calibration curves that came from studies of healthy volunteers. The red (660 nm) and infrared (940 nm) wavelengths are used to differentiate between reduced hemoglobin and oxyhemoglobin. Reduced hemoglobin absorbs more light at the red band (650750 nm) than oxyhemoglobin, and oxyhemoglobin absorbs more light in the infrared band (900-1000 nm) than reduced hemoglobin. Pulse oximetry measures SPO2, which is not the same as the arterial saturation (SaO2). SaO2 is measured in the laboratory using cooximetry. Laboratory cooximeters use multiple wavelengths to distinguish, by their absorption, different types of hemoglobin. In most patients the levels of these other hemoglobin types (ie, carboxyhemoglobin) are so low that the SPO2 will approximate SaO2. The oxyhemoglobin dissociation curve (Figure 1) displays the relationship that exists between oxygen tension (PO2) and the arterial saturation. There is a predictable correlation between SaO2 and PO2 only on the steep portion of this curve. At higher PO2 (> 75 mm Hg) there is a plateau in the SaO2, so that a relatively large change in PO2 there is very little corresponding change in SaO2. Figure 1. The oxyhemoglobin dissociation curve. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:672-673. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:14481453. 3. Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 4th ed. Baltimore: Williams & Wilkins; 1999:811-842. 50 4. Sinex JE. Pulse oximetry. Principles and limitations. Am J Emerg Med. 1999; 17:59-67. 5. McMorrow RC, Mythen MG. Pulse oximetry. Curr Opin Crit Care. 2006; 12:269-271. ITEM 51 Pulse oximeters are designed to interpret any pulsatile flow as arterial and will therefore assume the light absorbance is due to arterial blood. Significant pulsations of venous blood can lead to falsely low estimation of the SPO2. Severe tricuspid regurgitation, high airway pressures during mechanical ventilation, and use of an intraaortic balloon pump are all clinical scenarios when venous pulsations may be lead to erroneously low SPO2 readings. Dyshemoglobins such as carboxyheloglobin and methemoglobin are important because they are unable to carry oxygen and are not measured accurately by standard pulse oximetry. Carboxyhemoglobin is present in varying degrees as a result of tobacco smoke and pollution, however it can reach dangerous concentrations as a result of smoke inhalation. High carboxyhemoglobin concentrations have also been reported during the administration of volatile anesthetics in combination with excessively desiccated absorbent. The absorption spectrum of carboxyhemoglobin at 660 nm is very similar to oxyhemoglobin, which therefore causes a falsely high SPO2 reading. Methemoglobin is an oxidation product of hemoglobin and forms a reversible complex with oxygen that impairs unloading of oxygen to tissues. Methemoglobin absorbs light equally at both the 660 nm and 940 nm wavelengths, the saturation is reported as 85%. Accordingly, the impact of high levels of methemoglobin on the SPO2 depends on the SaO2. If SaO2 levels exceed 85%, the presence of methemoglobin on the SPO2 depends on the SaO2. If SaO2 levels exceed 85%, the presence of methemoglobin will result in the SPO2 underestimating the true value; however, at a low SaO2 (< 85%) the presence of methemoglobin will cause the SPO2 to be falsely high. The discrepancy between SPO2 and SaO2 increases as the level of methemoglobin increases. Newer multiwavelength oximeters are being developed that may be able to distinguish between various hemoglobinopathies noninvasively. Ambient light—especially fluorescent light—can lead to falsely increased SPO2 readings. There are several methods to minimize the effects of this type of interference such as shielding the probe from the light, using the correct sensor size for the patient, and making sure the sensor is properly positioned. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:672-673. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:14481453. 3. Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 4th ed. Baltimore: Williams & Wilkins; 1999:811-842. 4. Varon AJ. Methemoglobinemia and pulse oximetry. Crit Care Med. 1992; 20:1363-1364. 5. Vegfors M, Lennmarken C. Carboxyhaemoglobinaemia and pulse oximetry. Br J Anaesth. 1991; 66:625-626. 6. Sinex JE. Pulse oximetry. Principles and limitations. Am J Emerg Med. 1999; 17:59-67. 7. McMorrow RC, Mythen MG. Pulse oximetry. Curr Opin Crit Care. 2006; 12:269-271. 51 ITEM 52 The prostate contains a rich venous plexus capable of absorbing the irrigating solution with subsequent development of the transurethral resection of the prostate (TURP) syndrome. The amount of fluid absorbed increases with the duration of the surgical resection, the inexperience of the surgeon, and the height of the irrigating solution relative to the surgical field. The higher the position of the irrigating fluid, the more likely it is to enter the prostatic venous sinuses and cause problems. It is recommended that the solution be kept less than 60 cm above the level of the surgical field. Distilled water was used in the past as it is relatively cheap and allows for excellent visualization of the surgical field. However, its use was abandoned because of its potential for inducing marked dilutional hyponatremia and intravascular hemolysis. Currently, a glycine solution is typically used as the irrigating solution. Symptoms specific to glycine absorption, especially transient blindness, may also occur. TURP syndrome (or water intoxication syndrome) is a general term used to describe a large range of neurologic and cardiopulmonary symptoms that occur when irrigating solution is absorbed during the procedure. Respiratory distress occurs due to rapid volume expansion with pulmonary edema. Hyponatremia (which may be profound) is a consequence of hemodilution because of excess total body water with normal total body sodium. Under spinal anesthesia, hyponatremia may manifest initially as neurologic dysfunction (confusion, agitation, etc.), but these signs will be masked if general anesthesia is used. Treatment of TURP syndrome includes optimization of oxygenation and ventilation, circulatory support, and notification of the surgeon to allow for coagulation of bleeding vessels and expeditious termination of the procedure. Mild symptoms may be treated with intravenous furosemide, but severe symptoms in the setting of a serum sodium less than 120 mEq/L should be treated with hypertonic (3%) saline, often guided by central venous pressure monitoring. Use of hypertonic saline to treat hyponatremia is less likely to cause central pontine myelinolysis when the hyponatremia has developed rapidly. New resectoscopes, which use bipolar or laser technologies, have been introduced. These devices may reduce the incidence of TURP syndrome. REFERENCES 1. Malhotra V. Transurethral resection of the prostate. Anesthesiol Clin North America. 2000; 18:883-897. 2. Frasco PE, Caswell RE, Novicki D. Venous air embolism during transurethral resection of the prostate. Anesth Analg. 2004; 99:1864-1866. 3. Issa MM, Young MR, Bullock AR, et al. Dilutional hyponatremia of TURP syndrome: A historical event in the 21st century. Urology. 2004; 64:298-301. 4. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1018-1012. ITEM 53 The celiac plexus lies anterior to the aorta, innervates abdominal viscera, and contains sympathetic as well as parasympathetic fibers. Parasympathetic nerve fibers originate from the craniosacral portions of the central nervous system, while sympathetic fibers originate from the thoracolumbar spinal cord. The celiac plexus does not contain somatic fibers. Celiac plexus block is useful in the treatment of intractable pain due to abdominal malignancies, chronic pancreatitis, and other visceral pain syndromes. 52 The easiest approach to the celiac plexus is via a posterolateral route, with the patient lying in the prone position. Radiologic guidance is commonly used, and anatomic landmarks are the body of the T12 and L1 vertebrae and the inferior margin of the 12th rib. Diagnostic blocks are performed using local anesthetic agents; more definitive neurolytic blocks commonly use ethanol or phenol. Complications include aortic injury, intravascular injection, pneumothorax, subarachnoid or epidural injection, paralysis, retroperitoneal hematoma, and injury to viscera (eg, kidney). Side effects include diarrhea and hypotension. Hypotension, usually due to sympathetic block, occurs commonly and generally responds well to intravenous fluid therapy. REFERENCES 1. Cousins MJ, Bridenbaugh PO. Neural Blockade in Clinical Anesthesia and Management of Pain. 3rd ed. Philadelphia: Lippincott-Raven; 1998:463-468. 2. De Cicco M, Matovic M, Balestreri L, et al. Single-needle celiac plexus block: Is needle tip position critical in patients with no regional anatomic distortions? Anesthesiology. 1997; 87:13011308. ITEM 54 Myotonic dystrophy, a multisystem disease, is the most common myotonic syndrome. Severity of disease is generally related to age of onset—the earlier the onset of symptoms, the more severe the disease will become. Myotonia is the continued involuntary contraction of muscle groups; when present in a mild form, it may manifest as difficulty in releasing one’s grasp following a firm handshake. This manifestation commonly causes patients to present with complaints of “stiffness.” Progressive weakness, especially of distal muscles, results in weakness of the hands (sometimes presenting as inability to hold things) and foot drop. It is important to note that although the response to nondepolarizing muscle relaxants is generally normal, these agents may not reverse a myotonic episode. Succinylcholine may induce myotonic contractions— potentially even in infants who have not previously manifested myotonia—and could theoretically be associated with profound hyperkalemia in patients with severe myotonia. Because anticholinesterase agents may also induce a myotonic episode, most authorities advocate use of short-acting nondepolarizing neuromuscular blocking drugs that do not require reversal. Hypothermia, not hyperthermia, is also a potential trigger for a myotonic event. Treatment of a myotonic episode may include direct injection of local anesthetic into the muscle; regional anesthesia, while not efficacious in treating a myotonic event, is not contraindicated in patients with myotonic dystrophy. REFERENCES 1. White RJ, Bass SP. Myotonic dystrophy and paediatric anaesthesia. Paediatr Anaesth. 2003; 13:94-102. 2. Bhatt JR, Pascuzzi RM. Neuromuscular disorders in clinical practice: Case studies. Neurol Clin. 2006; 24:233-265. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:536537. 53 4. Baum VC, O’Flaherty JE. Anesthesia for Genetic, Metabolic, and Dysmorphic Syndromes of Childhood. Philadelphia: Lippincott Williams and Wilkins; 1999:212-214. ITEM 55 Although the presence of cyanosis in the first 24 hours of life is a feature in a long list of differential diagnoses, administration of 100% oxygen generally will distinguish a cardiac etiology from most other factors. A minimal-to-absent increase of PaO2 in response to administration of high inspired oxygen concentrations is strongly suggestive of the presence of a congenital cardiac lesion. Conversely, an increase in PaO2 to greater than 150 mm Hg effectively excludes an intracardiac shunt. Surfactant deficiency (Figure 1) is most commonly associated with prematurity and is manifested by tachypnea, retractions (suprasternal, intercostal, subcostal), grunting, nasal flaring, and hypoxemia. Auscultation of the chest reveals decreased lung volumes, a “ground glass” appearance, and air bronchograms. Even neonates with the most severe respiratory distress syndrome generally manifest an increase in PaO2 in response to administration of 100% oxygen. Figure 1. Radiograph of neonate with surfactant deficiency. The chest radiograph is commonly described as showing a reticulogranular or “ground glass” pattern. Air bronchograms are generally evident. Lung volume may be reduced (less than eight intercostal spaces on each side). In neonates the initial manifestation of sepsis is commonly tachypnea and peripheral cyanosis. Administration of 100% oxygen to a septic neonate generally results in a significant increase in PaO2. Transient tachypnea of the newborn (Figure 2) occurs primarily in term neonates, usually in association with maternal diabetes, cesarean delivery, or maternal asthma. Neonates most commonly present with tachypnea hypothesized to be secondary to delayed absorption of fetal lung fluid; retractions are uncommon. Hypoxemia, if present, is usually resolved with minimal increases in inspired oxygen 54 concentration. A characteristic chest radiograph demonstrates increased interstitial markings and fluid in the major fissure. Figure 2. A typical chest radiograph of a neonate with transient tachypnea of the newborn demonstrates a normal lung volume (eight intercostal spaces on each side). The presence of increased lung fluid is manifested by hazy lung fields, increased perihilar markings, and fluid in the fissure. REFERENCES 1. Sasidharan P. An approach to diagnosis and management of cyanosis and tachypnea in term infants. Pediatr Clin North Am. 2004; 51:999-1021. 2. Behrman RE, Kleigman R, Jenson HB. Nelson Textbook of Pediatrics. 17th ed. Philadelphia: WB Saunders; 2004:1523-1524. 55 ITEM 56 Clinical scenario: A patient undergoing general anesthesia initially received intravenous (IV) succinylcholine (100 mg) to facilitate tracheal intubation. Vecuronium was administered after the patient exhibited signs of recovery from succinylcholine. Towards the end of the procedure, neuromuscular blockade was reversed with neostigmine (5 mg IV) and glycopyrrolate (1 mg IV). However, prior to skin closure the patient began to move, and succinylcholine (20 mg IV) was administered. The patient subsequently experiences a prolonged weakness. Structurally, succinylcholine resembles two molecules of acetylcholine, and the drug is metabolized by plasma cholinesterase—an enzyme present in plasma. This enzyme has no other known function. Acetylcholine released at muscarinic and nicotinic cholinergic receptors is metabolized by (true) acetylcholinesterase, and enzyme strategically located in the immediate vicinity of the cholinergic receptors. Acetylcholinesterase does not metabolize succinylcholine. The residual effects of nondepolarizing neuromuscular blockers are reversed by administering inhibitors of acetylcholinesterase, such as neostigmine or pyridostigmine. The resultant increase in the concentration of acetylcholine results in a competitive displacement of nondepolarizing neuromuscular blockers from nicotinic acetylcholine receptors. Displaced nondepolarizing neuromuscular blockers then diffuse into plasma and undergo biotransformation and elimination. Patients with a low serum level of normal plasma cholinesterase usually do not manifest a prolonged block from succinylcholine. If this were the problem, it would have been manifested after the initial dose of succinylcholine. Recurarization occurs when nondepolarizing neuromuscular blocker effects have not been fully reversed. Effects of residual block are later potentiated from one of two mechanisms. This could occur with termination of the action of neostigmine (or another anticholinesterase) or the presence of conditions known to enhance the effects of nondepolarizing neuromuscular blockers (eg, respiratory or metabolic acidosis, hypothermia, or administration of drugs such as aminoglycosides). Neostigmine and pyridostigmine inhibit both plasma cholinesterase and acetylcholinesterase. The most likely etiology of weakness in this setting is from prolonged action of the second dose of succinylcholine due to inhibition of its metabolism. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:492. 2. Sunew KY, Hicks RG. Effects of neostigmine and pyridostigmine on duration of succinylcholine action and pseudocholinesterase activity. Anesthesiology. 1978; 49:188-191. ITEM 57 Propofol is a commonly used sedative hypnotic agent that acts via activation of GABAA receptors. Propofol is lipid soluble and must be supplied as a solution in a carrier emulsion. This emulsion consists of soybean oil, glycerol, and egg phosphatide. The emulsion supports bacterial growth and should be discarded if not used within six hours. Two formulations of propofol are available—Diprivan and generic. The preservatives and pH vary with the formulation. Diprivan contains disodium edentate (pH 7.0-8.5) as a preservative, while generic propofol contains sodium metabisulfite (pH 4.5-6.4). Although propofol itself has been shown to produce 56 bronchodilation, the emulsion formulation of generic propofol has been associated with bronchoconstriction, especially in patients with asthma; this has been attributed to the preservative metabisulfate. Severe metabolic acidosis has been reported primarily after long infusions of propofol. Previously it was thought that due to an increased risk of anaphylaxis patients with egg allergies should not receive propofol because of the presence of the egg phosphatide in the emulsion. However, most egg allergies are due to egg albumin not egg phosphatide. Therefore, propofol may be used in patients with egg allergies, but a heightened awareness for the development of allergic reactions should be maintained. Anaphylaxis has been reported after the first exposure to propofol; these patients tend to have histories of other allergies such as to neuromuscular blocking agents or multiple foods, including soy products. Adrenal suppression has been associated with the administration of etomidate, not propofol. REFERENCES 1. Marik PE. Propofol: Therapeutic indications and side-effects. Curr Pharm Des. 2004; 10:36393649. 2. Stoelting RK, Hillier SC. Pharmacology and Physiology in Anesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:155-162. ITEM 58 The Bainbridge reflex is one of several cardiac reflexes present to maintain cardiac output over a wide range of physiologic conditions. The Bainbridge reflex is elicited by stretch receptors located in the right atrium and the cavoatrial junction. The reflex is activated by right atrial distension. The physiologic response of the Bainbridge reflex involves the cardiovascular and central nervous systems. Increased right-sided heart pressures are sensed by the stretch receptors and signals are sent to the central nervous system, specifically to the medulla. The resulting decreased parasympathetic activity produces an increased heart rate. Tachycardia can also result from direct stimulation of the sinoatrial node due to stretching of the atrium. Hypertension, bradycardia, and hypotension are not manifestations of the Bainbridge reflex. REFERENCES 1. Barbieri R, Triedman JK, Saul JP. Heart rate control and mechanical cardiopulmonary coupling to assess central volume: A systems analysis. Am J Physiol Regul Integr Comp Physiol. 2002; 283:R1210-R1220. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:739. ITEM 59 There is no truer phrase than an “ounce of prevention is worth a pound of cure” when it comes to cardiac toxicity caused by local anesthetics (LAs). The use of a test dose and divided doses during injection are intended to reduce the risk of delivering a toxic dose by intravascular injection. In the event that LAinduced cardiac toxicity occurs, early detection and rapid intervention remain the cornerstones of a successful outcome. Advanced Cardiac Life Support (ACLS) measures should be instituted immediately as well as specific treatments for LA toxicity. 57 Treatment is directed at correcting cardiac dysrhythmias and myocardial depression. Because acidosis, hypoxia, and hypercarbia worsen LA-induced toxicity, particularly with bupivacaine, airway management is a key therapeutic intervention. Prevention of acidosis, both metabolic and respiratory, will minimize ion tapping, thereby decreasing the concentration of amide local anesthetics within the cells, including the cardiac myocytes. Early consideration for cardiopulmonary bypass should be given as bupivacaineinduced dysrhythmias can persist for long periods. Hypotension due to reduced contractility should be treated. Most animal studies support the use of sympathomimetics such as epinephrine and norepinephrine. Epinephrine, however, is not without risk as it can worsen dysrhythmias associated with LA toxicity. Recently the use of phosphodiesterase inhibitors, such as amrinone and milrinone, has been studied—with mixed results. The ACLS guidelines now recommend using vasopressin in place of, or in addition to, epinephrine. This requires further study to evaluate its efficacy in the treatment of LA-induced toxicity. Treatment of LA-induced dysrhythmias is recommended although there is not a specific agent that has demonstrated efficacy. Amiodarone is the primary drug in the ACLS dysrhythmia treatment algorithm. Some case reports have discussed the use of bretylium, and animal studies have demonstrated its efficacy. However, bretylium is no longer available in North America or Europe. Calcium channel blockers should be avoided in the setting of LA-induced toxicity. Animal studies have demonstrated an increased mortality associated with their use. Recent animal studies have demonstrated the effectiveness of lipid emulsion infusions in resuscitation of bupivacaine-induced cardiac toxicity. The mechanism is under investigation but is thought to be due to migration of amphophilic LA from binding sites in the myocardium into the plasma-rich lipid. It should be noted that there are only a few case reports of successful resuscitation of LA toxicity using intravenous lipid infusions. Novel therapies that are under investigation include the use of propofol or insulin/glucose infusions. REFERENCES 1. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:XXXX. 2. Tetzlaff JE. Clinical Pharmacology of Local Anesthetics. Boston: Butterworth-Heinemann; 2000:41-45. 3. Litz RJ, Popp M, Stehr SN, et al. Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia. 2006; 61:800-801. 4. Corcoran W, Butterworth J, Weller RS, et al. Local anesthetic-induced cardiac toxicity: A survey of contemporary practice strategies among academic anesthesiology departments. Anesth Analg. 2006; 103:1322-1326. 5. Weinberg GL. Current concepts in resuscitation of patients with local anesthetic cardiac toxicity. Reg Anesth Pain Med. 2002; 27:568-575. ITEM 60 Acute lung injury (ALI) and acute respiratory syndrome (ARDS) are disorders of the respiratory system characterized by the rapidly progressive onset of severe hypoxemic respiratory failure in the absence of cardiac failure or volume overload. Mortality rates from ALI and ARDS approach 50%. 58 The treatment of ALI/ARDS is largely supportive and includes addressing the underlying cause, optimization of mechanical ventilation, careful management of fluid balance, aggressive nutritional support, and prevention of other concurrent medical complications. In 2000, the Acute Respiratory Distress Syndrome Network (ARDSNet) published the results of a large prospective, randomized, multicenter trial that demonstrated a 22% reduction in patient mortality with a low tidal volume ventilation strategy (6 mL/kg vs 12 mL/kg calculated on the basis of ideal body weight). The authors hypothesized that this strategy reduces ventilator-associated lung injury from alveolar stretch (volutrauma) and decreases the release of inflammatory mediators (biotrauma) that might worsen lung function in the remaining noninjured lung. Since the publication of this study, many intensive care units have incorporated this ventilation strategy to treat patients with ALI and ARDS. REFERENCES 1. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342:1301-1308. 2. Hemmila MR, Napolitano LM. Severe respiratory failure: Advanced treatment options. Crit Care Med. 2006; 34(9 Suppl):S278-S290. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1473-1498. ITEM 61 Severe sepsis is a syndrome characterized by systemic inflammation from an infectious source associated with acute organ dysfunction (ie, hypotension, respiratory failure, acute renal failure, or altered mental status). Because organ dysfunction may be related to microcirculatory thrombosis with subsequent reductions in organ perfusion, intense research has been focused on the biologic pathways governing microcirculatory coagulation. The goal of this work has been to improve morbidity and mortality by preventing or reversing organ dysfunction during the early stages of the severe septic episode. Protein C is an important mediator in both the inflammatory and coagulation pathways. In its activated form, protein C inhibits microvascular thrombosis and inflammation while promoting fibrinolysis. In patients with severe sepsis, the production and activation of protein C is significantly reduced, resulting in a potent prothrombotic state. Activated drotrecogin alfa (DrotAA) with the trade name Xigris is a recombinant form of human activated protein C that has recently been shown to significantly decrease mortality in some patients with severe sepsis. Indications for DrotAA include a systemic inflammatory response, presumably due to infection, and evidence of dysfunction in at least one of the following associated organ systems: Cardiovascular (shock, hypotension, or vasopressor use) Respiratory (PaO2/FIO2 ratio < 250) Renal (oliguria despite adequate fluid resuscitation) Hematologic (thrombocytopenia) 59 Because DrotAA (Xigris) is also a potent anticoagulant, one of its most serious side effects is hemorrhage during drug infusion. During administration there is a 3.5% incidence of serious bleeding that occurs primarily in patients with an underlying predisposition to hemorrhage. Contraindications to DrotAA administration include: active internal bleeding recent (within three months) hemorrhagic stroke recent (within two months) intracranial or intraspinal injury or severe head trauma trauma with increased risk of life-threatening bleeding presence of an epidural catheter intracranial neoplasm or mass lesion or evidence of cerebral herniation REFERENCES 1. Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001; 344:699-709. 2. Bernard GR. Drotrecogin alfa (activated) (recombinant human activated protein C) for the treatment of severe sepsis. Crit Care Med. 2003; 31(1 Suppl):S85-S93. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1473-1498. ITEM 62 Cerebral vasospasm is an important cause of delayed morbidity and mortality in patients with subarachnoid hemorrhage following intracranial aneurysm rupture. Clinically important vasospasm typically occurs between 4 and 12 days after subarachnoid hemorrhage and is often associated with systemic hypertension, headache, confusion, lethargy, and focal motor/sensory deficits. Cerebral vasospasm is typically suspected based on transcranial Doppler ultrasonography; diagnosis is confirmed on the basis of cerebral angiography. Nimodipine, an oral calcium-channel blocker, is prophylactically administered to patients with subarachnoid hemorrhage to reduce the risk of this devastating complication. Traditional therapy for cerebral vasospasm generally includes “triple-H” therapy (hypervolemia, hypertension, and hemodilution). The goal of this therapy is to maximize regional cerebral blood flow by optimizing the systemic blood pressure using aggressive fluid resuscitation and vasopressors (phenylephrine, norepinephrine, and vasopressin) and decreasing blood viscosity through hemodilution to a hematocrit of 30%-33%. Additionally, superselective angioplasty and intraarterial injection of papaverine and verapamil can be performed at angiography to improve cerebral blood flow. Hyperventilation has no role in the treatment of cerebral vasospasm following subarachnoid hemorrhage. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:777-779. 2. Treggiari MM, Walder B, Suter PM, et al. Systematic review of the prevention of delayed ischemic neurologic deficits with hypertension, hypervolemia, and hemodilution therapy following subarachnoid hemorrhage. J Neurosurg. 2003; 98:978-984. 60 3. Feng L, Fitzsimmons BF, Young WL, et al. Intaarterially administered verapamil as adjunct therapy for cerebral vasospasm: Safety and 2-year experience. AJNR Am J Neuroradiol. 2002; 23:1284-1290. ITEM 63 During orthotopic cardiac transplantation the donor heart is surgically implanted into the recipient patient using a biatrial technique. During the operative procedure, the posterior portion of the recipient’s native heart is preserved and sutured to the donor heart. Because the sinoatrial (SA) node typically lies in this portion of the heart, the recipient’s SA node (with its central autonomic connections intact) may still be present following the surgical procedure along with the SA node from the donor heart (with its cardiac conduction system intact). Patients following cardiac transplantation, therefore, may have two distinct SA nodes that are electrically isolated from each other by an atrial suture line. In these situations, the electrocardiogram may demonstrate two P waves, with the native wave, isolated from the rest of the cardiac conduction system, dissociated from the QRS complex. The donor heart SA node is the functional pacemaker and transmits the electrical signal to the donor atrioventricular (AV) node and the ventricles. The functional pacemaker in the transplanted heart, therefore, receives no autonomic nervous system input from the central nervous system and typically has an intrinsic rate between 80 and 100 beats/min. The transplanted heart retains its sensitivity to beta-adrenergic agonists and antagonists and may, in fact, demonstrate supersensitivity to catecholamines. Therefore, medications with direct beta-adrenergic activity produce normal to increased activity on both the heart rate and contractility in the denervated heart. Medications that act indirectly through central nervous system sympathetic and parasympathetic autonomic nerve fibers have little or no effect. Epinephrine, norepinephrine, ephedrine, dopamine, dobutamine, and isoproterenol all have direct betaadrenergic activity and increase heart rate when administered to patients after heart transplant. Ephedrine, a mixed direct and indirect beta-adrenergic agonist, also increases the heart rate in patients following heart transplant. Atropine and glycopyrrolate, however, have little effect on the patient’s heart rate because the donor sinus node is electrically isolated from the central parasympathetic system. Phenylephrine has no chronotropic effect. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1370-1374. 2. Melton IC, Gilligan DM, Wood MA, et al. Optimal cardiac pacing after heart transplantation. Pacing Clin Electrophysiol. 1999; 22:1510-1527. 3. Cannom DS, Rider AK, Stinson EB, et al. Electrophysiologic studies in the denervated transplanted human heart. II. Response to norepinephrine, isoproterenol and propanolol. Am J Cardiol. 1975; 36:859-866. 4. Gilbert EM, Eiswirth CC, Mealey PC, et al. Beta-adrenergic supersensitivity of the transplanted human heart is presynaptic in origin. Circulation. 1989; 79:344-349. ITEM 64 Ventilator-associated pneumonia (VAP) is an important medical problem in patients receiving mechanical ventilation in the intensive care unit. It is generally defined as pneumonia developing 48-72 hours 61 following tracheal intubation, and it occurs in 10%-25% of patients intubated for more than 48 hours. VAP is associated with a 25% to 70% mortality rate. VAP occurs due to a combination of host and pathogen factors. In the host (patient), there is an abnormal conduit (tracheal tube) from the upper airways to the lower airways, bypassing the normal protective mechanisms. This conduit allows secretions from the oropharynx to pool just above the tracheal tube cuff. Small amounts of contaminated secretions can then be aspirated around the cuff during spontaneous inspiration or suctioning. These aspirated, contaminated secretions are dispersed throughout the lung and may cause infections disease, such as pneumonia. Current published guidelines recommend several interventions to decrease the incidence of VAP within the intensive care unit, such as avoidance of tracheal intubation (if possible) shortening the duration of mechanical ventilation use of continuous sublgottic suction (Figure 1 and Figure 2.) semi-erect patient positioning early tracheostomy Sucralfate administration, frequent ventilator tubing changes, and routine prophylactic antibiotic administration are relatively contraindicated as they are associated with increased VAP rates and select for pathogens resistant to multiple drugs. Figure 1. Tracheal tube with subglottic suction. The second connector is placed to continuous suction. 62 Figure 2. Close-up view of subglottic suction port. This port lies just above the tracheal tube cuff and serves to remove pooled secretions. These secretions are suctioned through a special channel in the tracheal tube wall to the connector shown in Figure 1. REFERENCES 1. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388-416. 2. Isakow W, Kolief MH. Preventing ventilator-associated pneumonia: An evidence-based approach of modifiable risk factors. Semin Respir Crit Care Med. 2006; 27:5-17. 3. Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: A meta-analysis. Am J Med. 2005; 118:11-18. 4. Murray MJ, Coursin DB, Pearl RG, et al. Critical Care Medicine: Perioperative Management. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002; 389-391. ITEM 65 The neuraxial blockade achieved with the administration of epidural local anesthetics is significantly affected by the addition of epinephrine. The duration of the block is increased. The alpha2-agonist effects of epinephrine result in a reduction in the dose or concentration of local anesthetic necessary to produce satisfactory analgesia in laboring parturients. 63 Despite the advantages of adding epinephrine to epidural local anesthetics, some anesthesiologists have avoided the routine use of epinephrine-containing solutions in obstetric patients because of the potential for adverse effects. In animal models, the intravenous administration of epinephrine has been shown to decrease uterine blood flow. However, the blood concentrations of epinephrine achieved with the epidural administration of epinephrine-containing local anesthetics are unlikely to produce a reduction in uterine blood flow. Doppler studies have confirmed that uteroplacental perfusion is unaffected by the addition of epinephrine to epidural local anesthetics for labor analgesia. Concerns about the effects of epidural epinephrine on umbilical vascular resistance have also been raised. In healthy fetuses, umbilical cord blood flow and umbilical vascular resistance are not affected by the epidural administration of epinephrine. In a fetus with increased baseline umbilical vascular resistance, the effect of epinephrine administered via an epidural is controversial. Some data suggest it might increase umbilical vascular resistance in this situation. The epidural administration of a local anesthetic with epinephrine 1:200,000 is associated with a transient decrease in uterine activity although the clinical significance is likely minimal. The effect is dosedependent. In fact, epinephrine concentrations of 1:300,000 and lower have not been found to affect uterine contractility. REFERENCES 1. Chestnut DH. Obstetric Anesthesia: Principles and Practice. 3rd ed. Philadelphia: Elsevier Mosby; 2004:198. 2. Niemi G. Advantages and disadvantages of adrenaline in regional anesthesia. Best Pract Res Clin Anaesthesiol. 2005; 19:229-245. ITEM 66 Found on the actin filament of cardiac muscle, troponin is required for normal cardiac and skeletal muscle contraction. The troponin complex consists of three units: troponin-I (cTnI), troponin-T (cTnT), and troponin-C, of which only the cTnI and cTnT units have cardiac specificity. Elevations in either cTnI or cTnT begin four to six hours after the onset of injury to cardiac myocytes and are very specific for the diagnosis of myocardial damage. They do not provide information as to the mechanism for the insult, however. Peak values occur 18-24 hours after the onset of the injury. Cardiac troponin concentrations may be elevated following a variety of situations including acute coronary syndromes, cardiac surgery, percutaneous coronary interventions, cardiac trauma, pulmonary embolism, and heart failure. The degree of increase provides prognostic information. Mortality rates for patients with acute coronary syndromes are more than doubled when peak cTnI concentrations increase from less than 5ng/mL to 9 ng/mL or higher. In these patients, an elevated troponin level has been found to be an independent predictor of 30-day mortality. Cardiac surgery with or without the use of cardiopulmonary bypass has the potential to cause damage to cardiac myocytes; in uncomplicated procedures, however, the increase of cardiac troponins should be minimal. Intraoperative events that can be expected to produce increases in troponin include cardiac manipulation, placement of cannulae, placement of pacing wires, and defibrillation. Adverse events that would cause greater increases in cardiac troponins include inadequate cardioplegia delivery, graft occlusion, or technically poor anastomoses. Increases in troponin concentrations following cardiac surgery do not provide information regarding the cause of the injury. The extent of the troponin increase following cardiac surgery, however, has been found to be indicative of the extent of myocardial damage and correlated to the prognosis. 64 REFERENCES 1. Babuin L, Jaffe AS. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ. 2005; 173:1191-1202. 2. Baggish AL, MacGillivray TE, Hoffman W, et al. Postoperative troponin-T predicts prolonged intensive care unit length of stay following cardiac surgery. Crit Care Med. 2004; 32:1866-1871. 3. Provenchere S, Berroeta C, Reynaud C, et al. Plasma brain natriuretic peptide and cardiac troponin I concentrations after adult cardiac surgery: Association with postoperative cardiac dysfunction and 1-year mortality. Crit Care Med. 2006; 34:995-1000. 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:733734. ITEM 67 Propofol infusion syndrome is a serious disorder that is most commonly associated with long-term infusion of propofol (> 48 hours) at high doses to provide sedation in critically ill patients. The syndrome is associated with otherwise unexplained metabolic acidosis rhabdomyolysis progressive cardiac failure Other abnormalities frequently reported in patients diagnosed with the disorder include renal failure, hyperkalemia, cardiac dysrhythmias, and lipemic serum. Liver failure has not been described as a clinical feature of the syndrome. The first published cases of propofol infusion syndrome occurred in children. However, there have now been several cases reported in adults. Although the majority of patients who have developed the syndrome were receiving high doses (> 4 mg · kg-1 · h-1) of the drug for long-term sedation, there have been reports of the syndrome occurring in patients who only received propofol for short-term anesthesia. In addition, other patients who have received short-term infusions have developed what might be considered a prodrome of the disorder—lactic acidosis with no other obvious causative factor. The pathophysiology of propofol infusion syndrome remains unknown. Current evidence supports a subcellular mechanism, as depicted in Figure 1, that involves failure of both the mitochondrial respiratory chain and metabolism of free fatty acids. This would result in low energy production, leading to cardiac and peripheral muscle necrosis. The mortality from propofol infusion syndrome is high. Among published cases in children, the mortality rate has been 73%. A recent review of deaths reported to the Food and Drug Administration in which propofol administered for long-term sedation was suspected of being a contributing factor found that 70% of pediatric deaths and 30% of adult deaths described a clinical presentation consistent with propofol infusion syndrome. Because this is such a serious disorder with a poor prognosis, some experts have made recommendations concerning the administration of propofol infusions in critically ill patients based on analysis of the published case reports. The American College of Critical Care Medicine recommends that practitioners consider utilizing another sedative medication if a patient receiving a high-dose propofol infusion develops cardiac failure or requires an increasing dose of vasopressor or inotrope. 65 Figure 1. Propofol increases the activity of malonyl coenzyme A (not shown), which in turn inhibits ( ) the carnitine palmitoyl transferase I (CPT I), so that long-chain FFA cannot enter the mitochondrion. Propofol also uncouples ( ) β-spiral oxidation and respiratory chain at complex II; therefore, neither medium- nor short-chain FFA that freely cross the mitochondrion membranes can be utilized. Low energy production can lead to cardiac and peripheral muscle necrosis if energy demand is high. (CoA coenzyme A, CoQ coenzyme Q, Cyt C cytochrome C, I, II, III, IV, V complexes of the respiratory chain). Adapted, from Vasile B, Rasulo F, Candiani A, et al. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003; 29:1417-1425. REFERENCES 1, Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:326. 2. Vasile B, Rasulo F, Candiani A, et al. The pathophysiology of propofol infusion syndrome: A simple name for a complex syndrome. Intensive Care Med. 2003; 29:1417-1425. 66 3. Wysowski DK, Pollock ML. Reports of death with use of propofol (Diprivan) for nonprocedural (long-term) sedation and literature review. Anesthesiology. 2006; 105:1047-1051. ITEM 68 Flow is the quantity of fluid (gas or liquid) that passes a point per unit time. The flow in smooth tubes at low rates of flow is laminar (there are no eddies or areas of turbulence.) The determinants of flow in a tube at laminar flow are summarized by the Hagen-Poiseuille equation: Flow = (P1-P2) π r4 8ηl where P1-P2 (or delta P) is the pressure gradient from one end of the tube (catheter) to the other, r is the mean radius of the tube, η (eta) is the viscosity of the fluid, and l is the length of the tube. Flow is related to the fourth power of the radius (diameter/2) of the catheter lumen. Flow increases as the radius increases so that a 14 gauge catheter, which has a wider radius than a 20 gauge catheter, will allow much more flow than the smaller catheter. Thus doubling the radius of the catheter will result in a 16-fold increase in flow rate. An increase in the catheter length leads to a decrease in the flow through the catheter. Accordingly, flow through the 14 gauge lumen of a standard 20 com triple-lumen central venous catheter will not be as high as flow through a peripherally inserted short 14 gauge catheter. Flow is inversely related to the viscosity of the fluid. Therefore, an increase in viscosity will lead to a decrease in flow. All other things being equal, crystalloid will flow more quickly through an intravenous catheter than will packed red blood cells. The inclusion of the term P1-P2 in the Hagen-Poiseuille equation gives a scientific basis to the practice of using pressure bags or rapid infusion devices for rapid administration of fluid. Flow is directly proportional to pressure differential and doubling the pressure differential will double the flow rate. REFERENCE 1. Davis PD, Kenny GNC. Basic Physics and Measurement in Anaesthesia. New York; Butterworth-Heinemann; 2003:11-22. ITEM 69 Helium is less dense than air or oxygen, though its viscosity is higher than that of air and lower than that of oxygen. Helium is an inert gas with no clinically significant tissue effects. Administration results in a decrease in resistance in the large airways secondary to a decrease in turbulent flow. Heliox is a blended mixture of oxygen and helium and is available in three ratios of helium to oxygen: 80:20, 70:30, and 60:40. The gas mixture may be useful in potentially reversible upper airway obstruction (eg, postextubation stridor) and in asthma, where it decreases both inspriratory and expiratory resistance. While an improvement in overall gas flow may improve oxygenation, the relatively low concentrations of oxygen in the mixture are a disadvantage if the patient is severely hypoxemic. Heliox may be delivered by mask or directly into a ventilator circuit. Spontaneously breathing patients may use a face mask with a reservoir bag. Relatively high flow needs to be delivered to ensure the bag remains full. Aerosolized medications are delivered through a Y-piece attached to the mask. 67 REFERENCES 1. Parrillo JE, Dellinger RP. Critical Care Medicine: Principles of Diagnosis and Management in the Adult. 2nd ed. St. Louis: Mosby; 2002:730. 2. MacIntyre NR, Branson RD. Mechanical Ventilation. Philadelphia: WB Saunders; 2001:454-457. 3. Hess DR, Fink JB, Venkataraman ST, et al. The history and principles of heliox. Respir Care. 2006; 51:608-612. 4. Jolliet P, Tassaux D. Usefulness of helium-oxygen mixtures in the treatment of mechanically ventilated patients. Curr Opin Crit Care. 2003; 9:45-50. 5. Fink JB. Opportunities and risks of using heliox in your clinical practice. Respir Care. 2006; 51:651-660. ITEM 70 Breathing systems (Figure 1) were classified by Mapleson into classes A to E in 1954. Willis subsequently added Class F. Typically only systems A, D, E, and F and their modifications are used during anesthesia, though systems B and C may be used in recovery and emergency situations. The systems have varying degrees of efficiency during spontaneous and controlled breathing. Figure 1. The range of Mapleson circuits. Used with permission, from Willis BA, Pender JW, Mapleson WW. Rebreathing in a T-piece: volunteer and theoretical studies of the Jackson-Rees modification of Ayre’s T-piece during spontaneous respiration. Br J Anaesth. 1975; 47:1239-1246. The Mapleson A system (also called the Magill system) is efficient for spontaneous ventilation as the fresh gas flow required is equal to the alveolar minute volume (approximately 70 mL · kg-1 · min-1). It is 68 inefficient for controlled ventilation as a fresh gas flow rate of three times the alveolar minute volume is required. The Mapleson D system is often used after a modification in which a small fixed tube inside a larger corrugated expiratory tube delivers fresh gas flow. This modified Mapleson D circuit is named the Bain circuit (see Figure 2). The Bain circuit is lightweight and compact at the patient end and may be useful where access to the patient is limited such as during head and neck surgery. For controlled ventilation 70 mL · kg-1 · min-1 will maintain normocapnia, although three times the patient’s minute ventilation is required during spontaneous ventilation. If the inner tube becomes kinked, leaks, or is dislodged, hypercapnia will ensue. Figure 2. The Bain circuit. Used with permission, from Faust RJ. Anesthesiology Review. 3rd ed. New York: Churchill Livingstone; 2002: figure 174.3, page 424. Mapleson systems E and F are valveless T-piece systems. They are typically used for anesthesia in children and are suitable for both spontaneous and controlled ventilation. A fresh gas flow rate of two or three times the minute volume and a minimum flow of 4L/min are required to prevent rebreathing of carbon dioxide. The doubled-ended bag in the Jackson Rees modification acts as a visual monitor during spontaneous ventilation and can be used for spontaneous or controlled ventilation as well as provide a degree of continuous positive airway pressure if required. REFERENCES 1. Morgan GE, Mikhail MS, Murray, et al. Clinical Anesthesiology. 3rd ed. New York: McGrawHill; 2001:29-33. 2. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:578-582. 3. Al-Shaikh B, Stacey S. Essentials of Anaesthetic Equipment. New York: Churchill Livingstone; 2002:40-47. 4. Faust RJ. Anesthesiology Review. 3rd ed. New York: Churchill Livingstone; 2002:222-223, 423425. 69 ITEM 71 Pulmonary hypertension may be divided into primary (the least common) or secondary causes. Secondary pulmonary hypertension in adults occurs as a result of pulmonary or cardiac disease. Causes include: resistance to pulmonary venous drainage o left ventricular failure o constrictive pericarditis o mitral stenosis, mitral regurgitation resistance to pulmonary vascular flow o chronic obstructive pulmonary disease o restrictive lung disease o pulmonary resection o congenital heart disease (Eisenmenger syndrome) o pulmonary thromboembolism o pulmonary stenosis hypoventilation o neuromuscular disorders o obesity and pickwickian syndrome other o hypoxemia o acidosis o high altitude o intravenous drug abuse Treatments for pulmonary hypertension include continuous intravenous prostaglandins, endothelin antagonists, or phosphodiesterase inhibitors. In the acute setting, avoidance of hypoxemia, hypercapnia, and acidosis will minimize worsening of existing pulmonary hypertension. Inhaled nitric oxide has been used in persistent pulmonary hypertension of the newborn and may be useful in the acute setting in both children and adults. Concentrations of up to 80 parts per million may be needed in pulmonary hypertension. Milrinone, an inodilator, is also useful in acute heart failure with pulmonary hypertension. REFERENCES 1. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006; 114:14171431. 2. Fischer LG, Van Aken H, Burkle H. Management of pulmonary hypertension: Physiological and pharmacological considerations for anesthesiologists. Anesth Analg. 2003; 96:1603-1616. 3. Blaise G, Langleben D, Hubert B. Pulmonary arterial hypertension: Pathophysiology and anesthetic approach. Anesthesiology. 2003; 99:1415-1432. 4. McGoon MD. The assessment of pulmonary hypertension. Clin Chest Med. 2001; 22:493-508. 5. Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998; 352:719-725. 6. Fischer LG, Van Aken H, Burkle H. Management of pulmonary hypertension: Physiological and pharmacological considerations for anesthesiologists. Anesth Analg. 2003; 96:1603-1616. 70 7. Lumb AB. Nunn’s Applied Respiratory Physiology. 6th ed. Philadelphia: Elsevier/Butterworth Heinemann; 2005:395-397. ITEM 72 Aprotinin is derived from bovine lung and inhibits serine proteases including plasmin and kallikrein. It has been found to reduce the risk of blood transfusion requirement in patients undergoing cardiac surgery. Desmopressin, a synthetic compound, is not derived from human blood. It is an analog of arginine vasopressin, an antidiuretic hormone that causes release of factor VIII and von Willebrand factor from vascular endothelium, transiently increasing their plasma levels. Recombinant activated factor VII (factor rVIIa) is made in tissue culture using baby hamster kidney cells. It can initiate coagulation at the site of vascular injury by binding with tissue factor and activating other coagulation factors (IX and X), resulting in thrombin formation and eventually clot. Tissue sealants are a group of hemostatic agents made from purified, virally inactivated human fibrinogen, human thrombin, and sometimes containing other components such as human factor XIII and bovine aprotinin. These tissue sealants are also called fibrin sealants or fibrin glue and have been used by cardiovascular surgeons to apply to suture lines and anastomoses to form stable fibrin clot at the sites where it is needed by reproducing the final stages in the coagulation cascade. In the United States, these agents are commercially marketed as Tisseel and Hemaseel. REFERENCES 1. DiDomenico RJ, Massad MG, Kpodonu J, et al. Use of recombinant activated factor VII for bleeding following operations requiring cardiopulmonary bypass. Chest. 2005; 127:1828-1835. 2. Levi M, Peters M, Buller HR. Efficacy and safety of recombinant factor VIIa for treatment of severe bleeding: A systematic review. Crit Care Med. 2005; 33:883-890. 3. Evans LA, Morey AF. Hemostatic agents and tissue glues in urologic injuries and wound healing. Urol Clin North Am. 2006; 33:1-12. 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:1117, 1974-1975. ITEM 73 Human recombinant antithrombin III can be used in the treatment of heparin resistance to provide the level of anticoagulation required for procedures utilizing cardiopulmonary bypass. It is a transgenic form of antithrombin III, meaning that it is made by inserting human DNA into goats, then isolating it from the milk of the goats’ cloned female offspring. Transgenic antithrombin III is free from any possible viral contamination and is more cost-effective than the antithrombin III isolated from human plasma. Several studies have shown that patients exhibiting heparin resistance, defined by activated clotting time (ACT) of less than 380 seconds after receiving 400 U/kg intravenous heparin, have benefited from the use of human recombinant antithrombin III. The increase in ACT that results is at least comparable to the increase achieved by the administration of fresh frozen plasma and can be achieved without blood product administration and the possibility of viral transmission. 71 Human recombinant antithrombin III is also being investigated for use in patients with lung injury due its effects on thrombin inhibition. Human recombinant antithrombin III is not used for patients with heparininduced thrombocytopenia, von Willebrand disease, or platelet dysfunction. The treatment for heparininduced thrombocytopenia is substitution of an alternative anticoagulant (eg, lepirudin, argatroban, danaparoid). Desmopressin is used for the treatment of von Willebrand disease. REFERENCES 1. Avidan MS, Levy JH, Scholz J, et al. A phase III, double-blind, placebo-controlled, multicenter study on the efficacy of recombinant human antithrombin in heparin-resistant patients scheduled to undergo cardiac surgery necessitating cardiopulmonary bypass. Anesthesiology. 2005; 102:276-284. 2. Aytekin FO, Tekin K, Kabay B, et al. Antithrombin III attenuates pulmonary tissue injury caused be mesenteric ischemia-reperfusion. Am J Surg. 2005; 189:161-166. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:19721974. ITEM 74 Attempts to identify a bedside physical examination that reliably predicts a difficult intubation have not been particularly successful. An editorial in Anesthesiology suggested that expecting an airway examination in a normal-appearing patient to predict a difficult intubation is a futile exercise. Difficult intubation is generally considered a grade 3 or 4 Cormack-Lehane view (Figure 1) of the larynx. Bedside examinations that have been proposed to predict difficult intubation include thyromental distance, sternomental distance, mouth opening, and more recently the mandible protrusion test. The mandible protrusion test consists of asking the patient to protrude his or her lower jaw in front of the maxilla. The distance between the upper teeth and the lower teeth is used to assess the ability to disarticulate the jaw and view the larynx. Figure 1. The modified diagrams of Cormack and Lehane used to document view at laryngoscopy. Used with permission, from Rose DK, Cohen MM. The incidence of airway problems depends on the definition used. Can J Anaesth. 1996; 43:30-34. A recent meta-analysis attempted to identify a single bedside test or combination of tests that reliably— with high sensitivity and high specificity—predicts the unexpected difficult intubation in normal appearing adults by combining the data from the best-designed studies. For a bedside examination to be 72 included in this analysis, three or more publications that met inclusion criteria needed to be available. Points that are important to consider when appraising any meta-analysis include the rigor of the search, the criteria for inclusion, and possible bias in the inclusion criteria. In this meta-analysis, 3,318 studies were found between 1980 and 2004 but only 35 (representing 50,760 patients) met the following established criteria: prospective study one or more bedside diagnostic tests were used data allowed calculation of true-positive, false-positive, true-negative, and false-negative results a standard laryngoscope was used It is important to note that the definition of “difficult to intubate” can vary among clinicians; the criteria in the meta-analysis for difficult intubation were a grade 3 or 4 Cormack-Lehane view or more than three attempts at intubation. The Mallampati score is used to assess the size of the tongue relative to the oral cavity. Some authors have suggested that tongue protrusion during Mallampati assessment indicates the degree to which the tongue can be displaced by the laryngoscope blade. The tongue must be displaced to obtain an unobstructed view of the larynx. Since the maneuver is often performed during head extension, Mallampati score may also indicate the degree of head and neck mobility. Poor mobility often lowers the Mallampati score. Despite the extensive nature of the examination, as a single test it has only a modest likelihood of predicting a difficult intubation (likelihood ratio 3.7; range 3.0-4.6). In addition, there was a wide range of predictive values among the included studies, probably due to the heterogeneity of the execution of the examination (eg, with or without phonation and/or with different head or tongue positions). Thyromental distance indicates mandibular space and reflects the amount of space available to displace the tongue during laryngoscopy. The threshold measurement that accurately predicts difficult intubation was not clear; the studies included in the meta-analysis predicted difficult intubation when minimal thyromental distance ranged between less than 4 to 7 cm. The various methods used for measurement (rule or fingerbreadths) contributed to the lack of clarity. Consequently, as a single test thyromental distance does not predict with adequate specificity or sensitivity difficult intubation (likelihood ratio 3.4; range 2.3-4.9). No “threshold value” could be suggested to predict a difficult intubation. Sternomental distance, measured by fully extending the neck, is a good measure of head and neck mobility, a factor believed to determine the ease or difficulty of intubation. As a single test, it had the best likelihood ratio (5.7; range 2.1-15.1) with acceptable sensitivity and specificity. The ease and consistency of measurement may contribute to the reliability of this test. However, again, a threshold predictor is not possible, in part because of the variation in patient size. In addition, a sternomental distance/patient height ratio to adjust for variation in stature was not performed. Finally, mouth opening is often used as an absolute predictor of difficult intubation. Mouth opening allows assessment of the movement of the tempromandibular joint. Clearly, when a patient cannot open his or her mouth even a centimeter visualization of the larynx with a standard scope will be difficult if not impossible. Several studies have indicated that limited mouth opening is strongly associated with difficult intubation. However, in the scenario of a “normal” appearance, the size of the mouth opening that predicts difficulty is extremely subjective, to the point of making the exam an inadequate predictor of difficult intubation. The likelihood ratio (4.0; range 2.0-8.2) is marginally better than that of the Mallampati exam. Combining tests more accurately predicts the likelihood of difficult intubation. Combining the Mallampati test with the thyromental distance results in the best likelihood ratio (9.9; range 3.1-31.9) and 73 best probability of predicting a difficult intubation. Either test alone had approximately a 15% chance of correctly predicting a difficult intubation. However, the combination had a 34% probability of correctly identifying a difficult intubation. Thus, a “normal” airway examination remains difficult to describe, and patients remain at risk of having an unexpected difficult intubation. Consensus threshold criteria where each bedside test becomes abnormal are: Mallampati grade III, IV Thyromental distance less than 6.0 cm Mouth opening less than two finger breadths Sternomental distance less than between 12.5 and 13.5 cm. REFERENCES 1. Rose DK, Cohen MM. The incidence of airway problems depends on the definition used. Can J Anaesth. 1996; 43:30-34. 2. Shiga T, Wajima Z, Inoue T, et al. Predicting difficult intubation in apparently normal patients: A meta-analysis of bedside screening test performance. Anesthesiology. 2005; 103:429-437. 3. Yentis SM. Predicting difficult intubation: Worthwhile exercise or pointless ritual? Anaesthesia. 2002; 57:105-109. 4. Cattano D, Panicucci E, Paolicchi A, et al. Risk factors assessment of the difficult airway: An Italian survey of 1956 patients. Anesth Analg. 2004; 99:1774-1779. 5. Khan ZH, Kashfi A, Ebrahimkhani E. A comparison of the upper lip bite test (a simple new technique) with modified Mallampati classification in predicting difficulty in endotracheal intubation: A prospective blinded study. Anesth Analg. 2003; 96:595-599. 6. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:608-613. ITEM 75 Postoperative ileus is a disruption of bowel motility after abdominal surgery or injury. It is a significant contributor to postoperative discomfort and delays in nutritional intake, hospital recovery, and discharge. Additionally, early enteral feeding has been shown to reduce postoperative morbidity. Therefore, every effort should be made to reduce postoperative ileus and encourage early oral nutritional intake. Factors that contribute to postoperative ileus include local inhibitory sympathetic reflexes activated at the site of injury, release of local inflammatory factors and neurotransmitters (eg, nitric oxide, vasoactive intestinal peptide, and substance P), release of endogenous opioids and administration of opioids for postoperative analgesia. Local inhibitory sympathetic reflexes are activated due to incision through the abdominal wall and mechanical manipulation of the visceral contents. This is mediated via afferent somatic and splanchnic nerves. These sympathetic reflexes cause release of norepinephrine, which inhibits excitatory release of acetylcholine in the myenteric plexus (also called Auerbach’s plexus), leading to intestinal wall relaxation. Additionally, spinal sympathetic neural reflexes are activated further inhibiting gastrointestinal function. 74 Localized bowel inflammation during surgery can lead to impairment of bowel function. Inflammation within the bowel wall causes release of proinflammatory cytokines and chemokines. These agents attract and activate leukocytes, which release proinflammatory mediators. These mediators cause inhibition of the enteric smooth muscle function for extended periods postoperatively. Surgery results in release of endogenous opioids into the bloodstream. Additionally, exogenous opioids are given for perioperative analgesia. Opioids inhibit gastrointestinal motility and are a significant factor in the development and maintenance of postoperative ileus. Endogenous opioids (eg, met- and leuenkephalins, mu-endorphin, and dynorphin) that reside in the neurons of the submucosal and myenteric plexus impair gut motility by inhibiting release of acetylcholine from cholinergic neurons. Exogenously administered opioids have a similar function. Anesthesiologists can help reduce postoperative ileus by limiting the dosing of systemic opioids and the use of continuous thoracic epidural local anesthetic analgesia. Continuous thoracic epidural local anesthetic analgesia can block the sympathetic fibers that innervate the gut. These sympathetic fibers, which arise from the T5 through the L1 levels, inhibit gut activity. Local anesthetic blockade removes this inhibition, leading to a small contracted intestine that is more likely to have normal peristalsis. An additional benefit is that the surgical view is improved due to the contracted intestine. Using thoracic epidural analgesia with local anesthetics also leads to a reduction in the need for systemic opioids and therefore limits their inhibitory action on the gut. Furthermore, the systemic absorption of local anesthetics has been found to improve gut motility through yet unknown mechanisms. Blockade of abdominal wall motor efferent fibers, while improving surgical conditions, is not thought to contribute significantly to resolution of postoperative ileus. Thoracic epidural analgesia with local anesthetics has not been found to inhibit vasoactive intestinal peptide significantly. Finally, blockade of parasympathetic fibers would have the opposite of the desired effect. REFERENCES 1. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:353-384. 2. Holte K, Kehlet H. Postoperative ileus: A preventable event. Br J Surg. 2000; 87:1480-1493. 3. Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003; 362:1921-1928. ITEM 76 A retrobulbar block with local anesthetic provides anesthesia to the cornea, conjunctiva, and uvea by blocking the ciliary nerves. It also provides akinesia of the extraocular muscles by blocking cranial nerves III, IV, and VI. It is often combined with a separate block of the orbicularis muscle to provide anesthesia for anterior chamber, corneal, and lens procedures under local anesthesia. Preceding the block, local anesthetic eye drops are often instilled. Traditionally, the block was performed with the patient maintaining an upward and inward gaze, which provided the advantage of the patient looking away from the needle. Computed tomography scans of orbits revealed that his gaze put the optic nerve and its sheath and the ophthalmic artery at greater risk of perforation during the block. Consequently, patients are currently advised to maintain a primary or inferonasal gaze. With this gaze a 30-gauge needle is then directed at the interotemporal quadrant with the lower eyelid retracted. The needle is introduced tangentially to the globe through the conjunctiva to a depth of 1 cm. One mL of local anesthetic is then injected to provide analgesia prior to the needle insertion for the retrobulbar block. 75 The retrobulbar block is then performed with a 23-gauge, 3-cm, blunt needle with the patient using the same primary or inferonasal gaze. The needle is inserted at the same location as the previous injection and is directed toward the apex of the orbit. A pop may be felt as the needle enters the orbital muscle cone. After the needle is past the equator of the globe, the needle should be directed toward the inferior part of the superior orbital fissure and then aspirated. Typically, 2 to 4 mL of local anesthetic are injected and should provide satisfactory anesthesia. Following injection, intermittent digital pressure is applied for several minutes to distribute the local anesthetic and decrease intraocular pressure. The superior oblique is often not paralyzed during the retrobulbar block, leading the eye to intort when the patient is instructed to gaze downward. The peribulbar block has been gaining in popularity as many consider it easier, safer, and less painful that the retrobulbar block, although reliable data confirming this are lacking. The muscle cone is not entered during this block. Disadvantages of the peribulbar block compared to the retrobulbar block include the need for larger injectate volumes (6-8 mL) causing a possible increase in intraocular pressure, slower onset (5-10 minutes), the potential for globe perforation (very rare), and local anesthetic myotoxic effects on the inferior rectus muscle leading to vertical diplopia. Both blocks have been found to deliver good pain control during surgery and are superior to topical anesthesia alone. Patient preparation for the peribulbar block is similar to that described for the retrobulbar block, in one of the more common techniques, two injections are required. One if placed inferotemporally and the other superonasally, medial and below the supraorbital notch. A blunt, 23- to 27- gauge, 3-cm needle is directed just beyond the equator of the globe and 4-5 mL of local anesthetic are injected at each site following aspiration. Hyaluronidase may be added to promote the spread of local anesthetic, and the addition of epinephrine (1:400,000) promotes vasoconstriction and reduces bleeding. REFERENCES 1. Cousins MJ, Bridenbaugh PO. Neural Blockade in Clinical Anesthesia and Management of Pain. 3rd ed. Philadelphia: Lippincott-Raven; 1998:546-550. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:1079. 3. Friedman DS, Bass EB, Lubomski LH, et al. Synthesis of the literature on the effectiveness of regional anesthesia for cataract surgery. Ophthalmology. 2001; 108:519-529. ITEM 77 Retrobulbar blocks are associated with local and systemic complications. Local complications include perforation of the globe, retrobulbar hemorrhage, and optic nerve damage. Retrobulbar hemorrhage is the most frequent complication (1%-3%) and may be associated with systemic anticoagulation. While many surgeons recommend the patient avoid aspirin or anticoagulants before surgery, many elderly patients cannot do this without consequences. Recent studies suggest that with appropriate monitoring cataract surgery can be safely performed without discontinuing anticoagulation. Retrobulbar hemorrhage is often associated with pain and proptosis but not seizures. If sever, the patient’s intraocular pressure and central retinal artery pulsations should be carefully monitored. The oculocardiac reflex can also be triggered as a consequence. Therefore, electrocardiographic (ECG) monitoring should be present. Systemic complications are rare but potentially lethal and may require aggressive therapy. Consequently, patients should have an intravenous catheter in place, an oxygen facemask, and monitoring (ECG, blood pressure, SPO2) during the block procedure. While the amount of local anesthetic used during a retrobulbar or peribulbar block is not sufficient to cause toxicity if inadvertently injected into a vein, this is not true for arterial injection. Relatively small amounts of local anesthetic unintentionally injected into the ophthalmic artery can lead to almost immediate seizures due to retrograde flow into the internal carotid 76 and midbrain structures. The local anesthetic is thought to cause a preferential blockade of inhibitory synapses, as the excitatory synapses are more resistant to local anesthetic depression. Seizures then rapidly manifest and cardiopulmonary arrest can follow shortly thereafter. These effects are typically selflimiting but require immediate recognition and treatment. Stimulation of the oculocardiac reflex would typically lead to bradycardia, asystole, or other (junctional, nodal, ectopic atrial or ventricular) dysrhythmias with hypotension. Subdural spread of local anesthetic resulting from direct injection into the optic nerve sheath would lead to respiratory depression of arrest, confusion, agitation, and contralateral ophthalmoplegia. REFERENCES 1. Cousins MJ, Bridenbaugh PO. Neural Blockade in Clinical Anesthesia and Management of Pain. 3rd ed. Philadelphia: Lippincott-Raven; 1998:546-550. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:1709. 3. Moorthy SS, Zaffer R, Rodriguez S, et al. Apnea and seizures following retrobulbar local anesthetic injection. J Clin Anesth. 2003; 15:267-270. ITEM 78 The childhood manifestation of infection by varicella zoster (a subtype of herpes virus) is chickenpox; the most common adult manifestation is shingles, commonly referred to as a herpes zoster infection. The initial herpes zoster infection typically occurs during childhood and leads to a chronic dormant infection of the dorsal root ganglion. The virus can be reactivated during adulthood under a variety of circumstances, leading to shingles. Approximately 500,000 people in the United States experience reactivation of herpes zoster virus each year. While as many as 20%-30% of the population as a whole will develop this condition, it occurs in as many as 50% of patients living to age 85 or older. Factors that can lead to reactivation of herpes zoster virus include stress, surgical procedures, toxic chemicals, ultraviolet light, radiotherapy, trauma, malignancy, systemic corticosteroid therapy, HIV infection, and other immune system suppressors. Most patients who develop shingles experience unilateral pain in a dermatomal distribution prior to the appearance of the rash. The majority of patients complain of moderate to severe pain with the appearance of the rash. For most patients the episode is self-limiting, typically lasting between two and four weeks. Shingles is seen most commonly in the thoracic dermatomal distribution affecting one or more unilateral segments. The ophthalmic distribution of the trigeminal nerve is the second most common site for shingles. Regions such as the maxillary and mandibular division of the trigeminal nerve, cervical, brachial, and lumbar dermatomes are affected less commonly. The pain and rash associated with shingles can be both severe and rapid in onset. The primary goal during this time is to keep the patient comfortable and to shorten the duration of pain after the rash has healed. Use of a combination of antiviral agents, analgesics, and possibly sympathetic blocks and epidural steroids has been shown to reduce the duration of pain. REFERENCES 1. Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003; 36:877-882. 77 2. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004; 62:1545-1551. 3. Baron R. Post-herpetic neuralgia case study: Optimizing pain control. Eur J Neurol. 2004; 11(Suppl 1):3-11. 4. Dworkin RH, Schmader KE. The epidemiology and natural history of herpes zoster and postherpetic neuralgia. In: Watson CPN, Gershon AA, eds. Herpes Zoster and Postherpetic Neuralgia. 2nd ed. New York: Elsevier; 2001:39-64. ITEM 79 Postherpetic neuralgia, a chronic neuropathic pain condition typically defined as pain lasting more than three months after the onset of the shingles rash, is a common complication of shingles. Increasing age is associated with an increased risk of developing postherpetic neuralgia. The pain associated with postherpetic neuralgia is most commonly described as burning, stabbing, or lancinating in nature. Factors associated with an increased risk of developing postherpetic neuralgia are listed in Table 1. Table 1. Risk factors for the development of postherpetic neuralgia. PPV = positive predictive value; NPV = negative predictive value. Modified, from Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004; 62:1545-1551. Risk Factor PPV NPV Specificity Sensitivity Age ≥ 50 years 0.19 0.94 0.81 0.47 Age ≥ 60 years 0.20 0.92 0.62 0.62 Presence of prodrome 0.15 0.95 0.94 0.17 Severe acute pain 0.26 0.91 0.49 0.78 Severe rash 0.20 0.93 0.72 0.56 Female sex 0.17 0.90 0.64 0.50 In addition to the severe pain caused by ophthalmic division herpes zoster virus infection, serious eye complications can result, leading to keratitis, corneal ulceration, iritis, and potentially permanent blindness. Although antiviral therapy has been shown to decrease the duration of pain, it is not 100% effective in preventing the development of postherpetic neuralgia. REFERENCES 1. Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003; 36:877-882. 2. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004; 62:1545-1551. 78 3. Baron R. Post-herpetic neuralgia case study: Optimizing pain control. Eur J Neurol. 2004; 11(Suppl 1):3-11. 4. Dworkin RH, Schmader KE. The epidemiology and natural history of herpes zoster and postherpetic neuralgia. In: Watson CPN, Gershon AA, eds. Herpes Zoster and Postherpetic Neuralgia. 2nd ed. New York: Elsevier; 2001:39-64. ITEM 80 Clinical scenario: A 27-year-old, gravida 1, para 0 woman presents for initial prenatal care. An ultrasound examination confirms a viable 18-week pregnancy. The patient denies any symptoms or problems since becoming pregnant but has been previously diagnosed with Marfan syndrome. Marfan syndrome is a connective tissue disorder that is inherited as an autosomal dominant trait and has a prevalence of 2-3 per 10,000 individuals. The skeletal, cardiovascular, pulmonary, and ocular systems are most commonly affected. During pregnancy, the disease may also be associated with cervical incompetence, abnormal placentation, and postpartum hemorrhage. The greatest concern in parturients with Marfan syndrome, however, is the risk of cardiovascular complications. Cystic medical necrosis of the aorta is responsible for the cardiovascular abnormalities most commonly associated with the syndrome, including aortic root dilation, aortic insufficiency, and aortic dissection. Measurement of the aortic root diameter via echocardiography should be performed in all parturients with Marfan syndrome. Women with aortic root diameter greater than 4.0 cm are at increased risk of aortic dissection during pregnancy. The increased cardiac output of pregnancy plays a role in this increased risk by producing greater shear force on the aorta. A parturient with a dilated aorta should be counseled that she is at high risk for aortic dissection and maternal mortality. Obstetricians often recommend that termination of pregnancy be considered in these patients. Pregnant women without aortic root dilation or other significant cardiac involvement usually tolerate pregnancy well. However, aortic dissection has rarely been reported in women who had a normal aortic root before pregnancy. Therefore, all parturients with Marfan syndrome should be monitored for signs and symptoms of aortic dissection, and serial echocardiograms throughout pregnancy are recommended. Initiation of beta-blocker therapy during pregnancy should also be considered, especially in women with aortic root dilation. The degree of cardiac involvement often determines the mode of delivery in women with Marfan syndrome. Cesarean delivery is usually recommended for parturients with aortic root dilation in order to avoid the hemodynamic stresses of labor and vaginal delivery. However, successful vaginal delivery has been reported in women with dilated aortic roots. In women without aortic root dilation or other cardiac abnormalities, cesarean delivery is generally reserved for obstetric indications. Instrument-assisted vaginal delivery is often planed for women with Marfan syndrome, however, to minimize the cardiovascular stresses associated with pushing and the Valsalva maneuver. Liver disease is not associated with Marfan syndrome. Therefore, routine performance of liver function tests during prenatal evaluation is not necessary. REFERENCES 1. Datta S. Anesthetic and Obstetric Management of High-Risk Pregnancy. 3rd ed. New York: Springer Verlag; 2004:193-194. 2. Gordon CF 3rd, Johnson MD. Anesthetic management of the pregnant patient with Marfan syndrome. J Clin Anesth. 1993; 5:248-251. 79 3. Rossiter JP, Repke JT, Morales AJ, et al. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol. 1995; 173:1590-1606. ITEM 81 Clinical scenario: A 27-year-old, gravida 1, para 0 woman presents for initial prenatal care. An ultrasound examination confirms a viable 18-week pregnancy. The patient denies any symptoms or problems since becoming pregnant but has been previously diagnosed with Marfan syndrome. On echocardiogram (see Figure 1), the patient has an aortic root diameter of 4.6 cm, which has remained stable throughout pregnancy. She presents for an elective cesarean delivery. A parturient with Marfan syndrome and a dilated aortic root is at increased risk for aortic dissection (Figure 1). Minimization of aortic wall shear forces is essential to prevent dissection. Both peak aortic pressure and the rate of change in aortic pressure play a role in the degree of shear stress that develops. Therefore, maintenance of hemodynamic stability is an important component in the anesthetic management of a parturient with Marfan syndrome. Beta-blockade therapy should be maintained in the perioperative period to help minimize increases in blood pressure and myocardial contractility that may result from the stress response to surgery. If a parturient with Marfan syndrome and an enlarged aortic root presenting for cesarean delivery has not been receiving beta-blockers during than antepartum period, the anesthesiologist should consider the initiation of acute beta-blockade therapy. While some data suggest an association between maternal administration of drugs with pure beta-antagonist activity and fetal acidosis and/or bradycardia, the cardiovascular advantages to the mother would likely outweigh the fetal risks in this situation. Invasive monitoring is useful for maintaining light hemodynamic control. Intraarterial blood pressure monitoring is essential to achieve the meticulous blood pressure control required during anesthesia and surgery. Central venous pressure monitoring to accurately assess intravascular volume status should also be considered in these patients. Continuous epidural anesthesia is an excellent anesthetic technique for cesarean delivery in a patient with Marfan syndrome. Slow, intermittent dosing of the catheter allows a slow onset of sympathetic blockade, thus avoiding the larger hemodynamic changes sometimes associated with rapid development of a sympathetic block. Superior postoperative analgesia with epidural opioid and local anesthetic can also be achieved with this anesthetic technique. Should hypotension occur during epidural anesthesia, preferred treatment includes intravascular fluid therapy and small, incremental doses of phenylephrine. Ephedrine should be avoided in the parturient with Marfan syndrome and a dilated aortic root because of its positive inotropic effect. There are two reasons to not select a general anesthetic for this patient—increased maternal mortality associated with general anesthesia in parturients and increased wall stress/shear forces on the aorta associated with rapid sequence induction. The sudden increases in blood pressure that frequently occur during laryngoscopy and induction of general anesthesia could produce increases in aortic wall stress and sheer forces. Single-injection spinal anesthesia and combined spinal-epidural anesthesia would be less desirable techniques for the parturient with Marfan syndrome. An important component of anesthetic management is maintenance of hemodynamic stability and avoidance of sudden changes in blood pressure. Compared to a continuous epidural anesthesia technique, a single-injection spinal or a combined spinal-epidural anesthetic that utilizes a surgical anesthesia dose of intrathecal local anesthetic is more likely to produce rapid hypotension requiring treatment (with the potential for overcorrection and subsequent hypertension). 80 Figure 1. Transesophageal echocardiography of the ascending aorta in the long axis view revealing tortuosity and dilation. REFERENCES 1. Datta S. Anesthetic and Obstetric Management of High-Risk Pregnancy. 3rd ed. New York: Springer Verlag; 2004:193-194. 2. Gordon CF 3rd, Johnson MD. Anesthetic management of the pregnant patient with Marfan syndrome. J Clin Anesth. 1993; 5:248-251. ITEM 82 Hypertension is a common finding in the preoperative care unit. Assessing the risk of proceeding to surgery remains a difficult problem. Anesthesiologists are often confronted with patients and their surgeons who want to proceed with surgery despite alarmingly high blood pressure. Caregivers often assume the hypertension is related to anxiety and is not a long-term medical problem producing significant organ dysfunction. This issue is particularly true in patients who present with isolated systolic hypertension and/or pulse pressure hypertension, defined as a pulse pressure (systolic minus diastolic blood pressure) greater than 65 mm Hg. Table 1 describes the blood pressure categories currently defined by the Joint National Committee on Hypertension and the accepted blood pressure ranges used in medical literature. 81 Table 1. Classification of hypertension (all values in mm Hg). SBP, systolic blood pressure; DBP, diastolic blood pressure. Used with permission, from Aronson S, Fontes MI. Hypertension: A new look at an old problem. Curr Opin Anaesthesiol. 2006; 19:59-64. Category SBP DBP Optimal < 120 AND < 80 Normal < 130 AND < 85 High Normal 130-139 OR 85-89 Mild hypertension 140-159 OR 90-99 Moderate hypertension 160-179 OR 100-109 Severe hypertension > 180 OR > 110 Isolated SBP hypertension > 140 AND < 90 Isolated systolic hypertension—systolic blood pressure over 140 mm Hg with a diastolic blood pressure between 65 and 90 mm Hg—is the most common subtype of hypertension worldwide; it usually presents in patients over 50 years of age and is present in approximately 66% of individuals over 50 years of age. Isolated systolic hypertension, normal diastolic pressure does not reduce the increased risk of stroke. In contrast, isolated diastolic hypertension is more prevalent in individuals under 40 years of age where it is a predictor of later cardiovascular risk. Diastolic hypertension is usually associated with some degree of systolic hypertension. Pulse pressure (systolic minus diastolic blood pressure) greater than 65 mm Hg is considered a form of hypertension. Patients with pulse pressure hypertension may have diastolic hypotension, making the organs that are pressure-dependent (myocardium, kidneys, and central nervous system) for adequate perfusion more susceptible to hypoperfusion injury. The pathogenesis of pulse pressure hypertension is postulated to be the stiffening the major conducting vessels (eg, aorta, femoral, and carotid arteries) preventing the elastic expansion that dampens pressure peaks during systole and the recoil that increases blood flow and supports blood pressure during late systole and diastole. Hypovolemia will produce a greater decrease in blood pressure without the normal constriction of conducting vessels in response to decreases in volume. The possibility of aortic insufficiency should be considered in a patient with pulse pressure hypertension. REFERENCES 1. Aronson S, Fontes ML. Hypertension: A new look at an old problem. Curr Opin Anaesthesiol. 2006; 19:59-64. 2. Verdecchia P, Angeli F. Natural history of hypertension subtypes. Circulation. 2005; 111:10941096. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:478. 82 ITEM 83 Clinical scenario: A 56-year-old woman presents in the preoperative care unit with a blood pressure of 195/65 mm Hg. Repeat determinations are 175/55 mm Hg and 180/55 mm Hg. There is no other preoperative information available. The patient asks if there are any additional perioperative risks of proceeding with elective surgery. Assessing an individual patient’s risk of proceeding with surgery based only on blood pressure values in the preoperative care unit is very difficult. There are many perioperative studies that have found systolic hypertension and diastolic hypertension are independent factors that convey increased risk of myocardial ischemia, myocardial infarction, stroke, and renal injury. An examination of patients who require cardiac surgery found 67% have a history of some form of hypertension and those who have isolated systolic hypertension or pulse pressure hypertension (systolic blood pressure more than 65 mm Hg above diastolic blood pressure) are the most likely to have postoperative cardiovascular or renal complications. While it is generally agreed that poorly controlled hypertension confers risk, the type of hypertension and specific blood pressure value where the risk of complication begins to increase rapidly or reaches an unacceptable threshold (eg, 5% risk of stroke) has not been determined. This makes the decision to cancel or delay the case somewhat arbitrary and dependent on medical judgment and regional standards. Cardiac risk conferred by pulse pressure hypertension is generalized from large population studies or studies of patients with known coronary artery, vascular, or renal disease. In an international study of patients undergoing cardiac surgery, those with preoperative pulse pressure hypertension were twice as likely to have a stroke as those without pulse pressure hypertension, and for each 10 mm Hg increase in pulse pressure there was a 21% increase in stroke. Cardiac complications increased by pulse pressure hypertension included a 50% increase in congestive heart failure and a 200% increase in cardiac mortality. Longitudinal studies in ambulatory patients have found pulse pressure hypertension a better predictor of cardiac disease and stroke than either systolic or diastolic blood pressure, neither of which were correlated with severity of disease. Mortality was highest in patients with a systolic blood pressure above 160 mm Hg and a diastolic blood pressure below 70 mm Hg, producing a pulse pressure above 90 mm Hg. Isolated diastolic hypotension did not affect mortality rate. After cardiac surgery, the occurrence of renal injury (new onset insufficiency or failure requiring dialysis) in patients with pulse pressure hypertension was nearly twice that of patients without pulse pressure hypertension (8.6% vs 4.5%). Mortality caused by renal failure was three times higher when pulse pressure hypertension is present prior to surgery. The incidence of postoperative renal disease in noncardiac surgical patients with pulse pressure hypertension remains largely unknown because of the low incidence of the problem. Comorbid conditions that predispose patients to pulse pressure hypertension include: advanced age systolic hypertension glucose intolerance or diabetes mellitus coronary artery disease hyperlipidemia sedentary life style increased homocysteine serum concentrations (also associated with cardiac disease) autoimmune inflammatory responses 83 angiotensin II type 1 receptor polymorphism Many of these conditions have been found to be independent risk factors or risk factors associated with perioperative cardiac complications, renal complications, and death. There is little information available regarding this patient’s general medical condition. Simple bedside tests like an electrocardiogram, serum lipids, serum electrolytes, and a glucose determination could help define perioperative risk. The decision to seek more information now or delay the case to allow for investigation and treatment of a patient’s comorbid conditions depends on the anesthesiologist’s best medical judgment. REFERENCES 1. Aronson S, Fontes ML. Hypertension: A new look at an old problem. Curr Opin Anaesthesiol. 2006; 19:59-64. 2. Verdecchia P, Angeli F. Natural history of hypertension subtypes. Circulation. 2005; 111:10941096. 3. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean arterial pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000; 160:1085-1089. 4. Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998; 104:343-348. 5. Aronson S, Boisvert D, Lapp W. Isolated systolic hypertension is associated with adverse outcomes from coronary artery bypass grafting surgery. Anesth Analg. 2002; 94:1079-1084. ITEM 84 Clinical scenario: A 71-year-old Jehovah’s Witness is to undergo a three-level (L3-S1) anterior-posterior spinal fusion with removal and replacement of hardware. The surgeon consults you to help develop a perioperative plan that allows the surgery without transfusion. Many patients are reluctant to accept blood products during surgery, with the most prominent and largest group being Jehovah’s Witnesses. Many patients are afraid of the infectious diseases potentially transmitted by blood products (eg, hepatitis, cytomegalovirus, HIV, and bacterial, rickettsial, and parasitic infections). Faith-based objections such as with the belief by Jehovah’s Witnesses that Christians must “abstain from blood” (King James Bible, Acts 15:28, 29) usually mean a management strategy must be developed that excludes administration of human blood components. Establishing which blood products and drugs cannot be administered is the first step toward developing a management plan. It is important during these discussions to have a private interview where the realistic consequences of these prohibitions are clearly outlined. Developing a written contract that outlines your understanding is also important to establish patient trust and physician responsibilities. While many patients will not accept blood products produced by another human or removal and retransfusion of their own blood, they will often accept new recombinant drugs such as recombinant human erythropoietin (EPO) and recombinant factor VIIa (rFVIIa). These factors are currently produced by genetic alteration of bacteria and are viewed by many but not all Jehovah’s Witnesses as acceptable. This is a personal decision not a general religious doctrine. Use of EPO has become a mainstay of therapy in many of the 200 worldwide bloodless surgery centers. EPO therapy effectively increases red blood cell mass in all patient populations regardless of age, sex, or 84 coexisting diseases. To be effective, erythropoietin needs to be administered for one to three weeks prior to surgery. In order for patients to respond to EPO, they must have adequate iron stores. Even when patients begin therapy with adequate stores, their red blood cell production can quickly outpace iron availability. Iron supplementation in doses proposed for treatment of iron deficiency anemia are recommended. Red blood cell expansion begins within three days of therapy and approximately one unit of blood is produced within seven days; approximately five units in 28 days. This rapid expansion of blood volume can lead to hypertension, hypercoagulable states, and congestive heart failure in susceptible individuals. This is not a benign therapy, and careful monitoring is required if large red cell volume expansion is planned. Despite these drawbacks, EPO therapy is an effective method to reduce the risk of organ injury and death due to inadequate red cell mass. Recombinant drug therapies available to increase other cellular blood components include: recombinant interleukin-11 (to increase platelet count) granulocyte-macrophage colony-stimulation factor (to increase white blood cell count) These may prove useful in the future since dilutional coagulopathy contributes to uncontrolled blood loss and death. Prevention of postoperative infection clearly improves surgical outcome. Neither drug, although used in chemotherapy and thrombocytopenic disorders, has been shown to be an effective preoperative management strategy to reduce the need for transfusion. REFERENCES 1. Shander A. Surgery without blood. Crit Care Med. 2003; 31(12 Suppl):S708-S714. 2. Goodnough LT, Monk TG, Andriole GL. Erythropoietin therapy. N Eng J Med. 1997; 336:933938. 3. Goodnough LT, Shander A, Brecher ME. Transfusion medicine: Looking to the future. Lancet. 2003; 361:161-169. ITEM 85 Clinical scenario: A 71-year-old Jehovah’s Witness is to undergo a three-level (L3-S1) anterior-posterior spinal fusion with removal and replacement of hardware. The surgeon consults you to help develop a perioperative plan that allows the surgery without transfusion. On the day of surgery, previously discussed plans to manage intraoperative blood loss are reaffirmed. Fluids and blood conservation methods that are available to the anesthesiologist depend on the agreement established with the patient. The family needs to be involved to understand the contract made with the patient, and unless legally empowered to do so, they cannot change these decisions. Patients who are not Jehovah’s Witnesses have a wider range of blood conservation options. Cell Saver, autologous donation, and acute normovolemic hemodilution are possible. Table 1 suggests the number of units of whole blood that probably can be conserved with each technique. 85 Table 1. Estimated effect of blood conservation techniques on units of blood provided. Adapted from Shander A. Surgery without blood. Crit Care Med. 2003; 31(12 Suppl):S708-S714. Technique Preoperative Units of Blood Conserved Administer erythropoietin 2-3 Preoperative autologous donation 1-2 Intraoperative Decrease allowable hemoglobin 1-2 Acute normovolemic hemodilution 1-2 Cell salvage with Cell Saver techniques 1-3 Meticulous hemostasis 1-2 Administration of fibrinolytic drugs 1 Postoperative Restrict phlebotomy 1 Continued blood salvage 1 Jehovah’s Witnesses believe that all constituent components of whole blood (red blood cells, platelets, white blood cells, and plasma) that are removed from the body should be discarded. Adherents do not accept transfusion of predeposited (autologous) blood. Techniques for intraoperative blood collection for later administration using standard cell salvage equipment is likewise not acceptable. Some patients will allow acute normovolemic hemodilution where several units of blood are removed at the beginning of the procedure blood volume is replaced with standard crystalloid solutions the blood is retransfused at the end of the procedure but only when the blood remains in a continuous connection with the patient through intravenous tubing. This is a very effective technique to decrease the probability of a critically low red cell mass, especially when erythropoietin is used to increase the hematocrit artificially. The use of some blood products such as albumin, immune globulins, and hemophiliac preparations is not specifically prohibited by religious doctrine and the patient may decide his position on these fluids. Maintenance of intravascular volume with hetastarch colloid products (Hextend, Hespan) and crystalloid is always accepted. 86 Use of antifibrinolytics, tranexamic acid, aminocaproic acid, and aprotinin has been suggested as a technique to reduce blood loss. Aprotinin has been reported to reduce blood loss by approximately 3 units and aminocaproic acid has been reported to reduce blood loss by approximately 1.0-1.5 units when compared to control patients in complex spine surgery. Randomized control trials comparing all three drugs are not available. However, some Jehovah’s Witnesses will not accept aprotinin since it is a bovine blood derivative. This is a personal choice not a religious doctrine. All antifibrinolytics, which require preemptive administration, carry some risk of hypercoagulability. Other rescue therapies, therapies administered during the procedure to improve coagulation, include: desmopressin to improve platelet adhesiveness and platelet count recombinant factor VIIa (rFVIIa) to improve platelet adhesiveness and activation of the extrinsic coagulation pathway If rFVIIa is administered, it should be in the setting of an established severe coagulopathy, not prior to incision. However, it is advisable that clotting factors and platelet count be within 20% of normal for rFVIIa to be effective. Hypothermia is associated with coagulopathy, vasoconstriction, and reductions in perfusion making even inadvertent hypothermia undesirable. Ultimately, careful planning is the most effective method to conduct anesthesia and surgical management safely. Defining an acceptable red cell mass determined by the patient’s overall health is the first step. Patients with significant cardiac disease and possibly cerebrovascular and renal disease will need a higher hemoglobin concentration than healthy patients to maintain critical organ perfusion. What the critical levels of hemoglobin or hematocrit are in a given patient is very difficult to determine, but physician agreement on a minimum acceptable value below which the surgery will be stopped to allow patient recovery must be reached. When possible the planned surgery should be preemptively separated into smaller procedures. Just prior to each surgical step, an assessment of the red cell mass and patient stability is made. The surgery can then be stopped when the maximum acceptable blood loss is reached or when signs of inadequate tissue perfusion occur (eg, ST segment changes, maximum heart rate). REFERENCES 1. Shander A. Surgery without blood. Crit Care Med. 2003; 31(12 Suppl):S708-S714. 2. Goodnough LT, Shander A, Brecher ME. Transfusion medicine: Looking to the future. Lancet. 2003; 361:161-169. 3. Dixon JL, Smalley MG. Jehovah’s Witnesses: The surgical/ethical challenge. JAMA. 1981; 246:2471-2472. Available online at http://www.watchtower.org/library/hb/article_06.htm. Accessed February 2007. 4. Urban MK, Beckman J, Gordon M, et al. The efficacy of antifibrionlytics in the reduction of blood loss during complex adult reconstructive spine surgery. Spine. 2001; 26:1152-1156. ITEM 86 Clinical scenario: A 71-year-old Jehovah’s Witness is to undergo a three-level (L3-S1) anterior-posterior spinal fusion with removal and replacement of hardware. The surgeon consults you to help develop a perioperative plan that allows the surgery without transfusion. On the day of surgery, previously discussed plans to manage intraoperative blood loss are reaffirmed. At the end of the procedure, the patient’s hemoglobin is 5.5 gm/dL. 87 A hemoglobin (Hb) of 6 gm/dL is clearly above the minimum required to maintain adequate oxygen delivery in an anesthetized, paralyzed, ventilated patient. With the return of generalized muscle activity, central nervous system activity, rhythmic respiratory muscle activity, and postsurgical stress, a threefold increase in oxygen consumption should be anticipated, increasing the minimum hematocrit required to meet metabolic needs to approximately 21% or Hb of 6.5 gm/dL when 100% oxygen is administered. In a patient with possible cardiac disease and other major comorbid conditions, the Hb of 6 gm/dL may not provide adequate oxygenation to critical organs. Thus minimizing oxygen demand by providing postoperative sedation, paralysis, and ventilation is an effective interval therapy until the red cell mass increases. Gradual withdrawal of these therapies with close monitoring for inadequate oxygen delivery (eg, ST segment depression, confusion, decreased urine output) is important to recovery. Administration of erythropoietin and iron will accelerate red blood cell production. A general estimate of the time needed to recover red cell mass for each stage of surgery is four weeks. While this is a good long-term strategy, it will not solve the immediate problem of inadequate oxygen-carrying capacity. Synthetic hemoglobin substitutes have long been sought. There are two main groups of artificial O2 carriers: hemoglobin-based, both bovine and human, and perfluorocarbon emulsions. Products currently in development in phase III trials include: HemAssist: a diaspirin cross-linked hemoglobin rHb1.1 and rHb2.0: human recombinant hemoglobin HBOC-201: polymerized bovine hemoglobin-based O2 carrier PolyHeme: human polymerized hemoglobin Hemolink: hemoglobin raffimer MP4: maleimide-activated polyethylene glycol-modified hemoglobin Oxygent: perflubron emulsion No hemoglobin substitutes are available in the United States and only Oxygent, PolyHeme, and Hemopure are in phase III trials. Their side effects and toxicity have severely restricted development. This category of drug would not be a reasonable alternative in this situation. Those derived from human and bovine hemoglobin may not be acceptable to Jehovah’s Witnesses. Aprotinin is most useful when administered prior to hemorrhage. It would not reduce oxygen demand or increase oxygen-carrying capacity, but it could be used to reduce additional blood loss. Another effective drug for reducing continued blood loss would be recombinant factor VIIa. It has been documented to provided hemostasis when administered as a single bolus dose or as a bolus dose followed by a continuous infusion, particularly when adequate clotting factors and platelets are available. REFERENCES 1. Spahn DR, Kocian R. Artificial O2 carriers: Status in 2005. Curr Pharm Des. 2005; 11:40994114. 2. Shander A. Surgery without blood. Crit Care Med. 2003; 31(12 Suppl):S708-S714. 3. Herbert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999; 340:409-417. 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:31813182. 88 ITEM 87 Clinical scenario: A 38-year-old male presents to the preoperative anesthesia clinic for evaluation prior to elective ankle surgery. He has a seizure disorder that is well controlled with phenytoin. He also takes the herbal remedies Echinacea and ginseng on a regular basis. During his visit to the clinic he complains of diplopia. On physical examination, nystagmus is noted. Phenytoin toxicity most commonly presents with central nervous system signs and symptoms, including nystagmus ataxia diplopia vertigo Toxicity may occur when the plasma drug concentration exceeds 20 mcg/mL. Because phenytoin is metabolized by hepatic microsomal enzymes, toxicity sometimes results from a drug interaction when a patient’s concomitantly taking a medication that inhibits these enzymes. Since this patient’s symptoms are consistent with phenytoin toxicity, increasing the dose of phenytoin is not appropriate. The use of herbal remedies has become increasingly popular in the United States. Studies have estimated that 20% of adults who take a prescription medicine also use an herbal supplement. The majority of patients with chronic diseases have tried some form of alternative medicine. In a survey of patients undergoing elective surgery, nearly one third were currently using an herbal medication and 70% of those patients had not informed the anesthesiologist of their use. Because some of these supplements can produce cardiovascular effects, affect the activity of other drugs, or interfere with normal coagulation, it is imperative that anesthesiologists include questions about the use of herbal remedies during the preanesthetic evaluation. Echinacea is one of the most popular herbal remedies in the United States. Because of its reported immunostimulant properties, it is used primarily to prevent the common cold and flu or to alleviate their symptoms. This herb can produce inhibition of hepatic microsomal enzymes. Therefore, it could lead to drug toxicity in patients taking medications that are metabolized by these enzymes, such as phenytoin and phenobarbital. In a patient who is exhibiting signs of phenytoin toxicity, echinacea should be discontinued. Ginseng is another herb commonly used in the United States. It is believed to possess antioxidant effects and is also popular among athletes to increase energy levels. It does not produce diplopia and nystagmus and is not associated with phenytoin toxicity. Diplopia and nystagmus in this patient are consistent with phenytoin toxicity; therefore an ophthalmology consult is not the most appropriate action at this point in time. If the laboratory result does not support the diagnosis of drug toxicity, an ophthalmology consult may be indicated. REFERENCES 1. Kaye AD, Kucera I, Sabar R. Perioperative anesthesia clinical considerations of alternative medicines. Anesthesiol Clin North America. 2004; 22:125-139. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:605606. 89 ITEM 88 Clinical scenario: A 38-year-old male presents to the preoperative anesthesia clinic for evaluation prior to elective ankle surgery. He has a seizure disorder that is well controlled with phenytoin. He also takes the herbal remedies Echinacea and ginseng on a regular basis. During his visit to the clinic he complains of diplopia. On physical examination, nystagmus is noted. After appropriate treatment, the patient’s diplopia and nystagmus resolve. He presents for his ankle surgery. He has continued to take ginseng on a regular basis and recently began taking ginger. Since an increasing number of patients undergoing anesthesia are taking herbal medications, the anesthesiologist must know how to counsel patients concerning the use of these substances during the perioperative period. Because these remedies could affect clotting activity or interact with anesthetic agents and other drugs, it is recommended that herbals be discontinued before surgery. When the pharmacokinetic data for an herbal are known, a specific recommendation concerning the timing of discontinuation can be made. However, this information is not available for most herbal preparations. Therefore, it is generally recommended that herbal remedies be discontinued two weeks before elective surgery. If a patient has not followed this recommendation, it is usually acceptable to proceed with surgery, provided that the anesthesiologist and surgeon are aware of any side effects that could be associated with recent use of the herbal medicine. One of the greatest concerns associated with perioperative herbal use is the potential for increased bleeding. Some of these agents inhibit clotting through a variety of mechanisms, most commonly by decreasing platelet aggregation. The most frequently used herbal medications associated with clotting inhibition are: garlic ginkgo ginger ginseng saw palmetto grape seed extract bilberry Despite a possible association between herbal remedy use and increased bleeding, the American Society of Regional Anesthesia’s consensus statement on neuraxial anesthesia and anticoagulation states that use of herbal medications does not interfere with the ability to safely perform neuraxial anesthesia techniques. Although ginger and ginseng both my inhibit clotting, there have been no reports of epidural or spinal hematoma after neuraxial anesthesia in patients taking these herbal medications. Other adverse effects may be associated with the preoperative use of ginseng. It possesses hypoglycemic properties that could lead to clinically significant hypoglycemia in preoperative patients who have been fasting for extended periods. Ginseng can also produce hypertension that may be potentiated by the administration of drugs with vasoconstrictive or inotropic effects. Ginger is most commonly used to prevent nausea and vomiting, especially in patients with a history of motion sickness. As a result, some patients may specifically begin using this herbal medication during the preoperative period in an attempt to prevent postoperative nausea and vomiting (PONV). The available data about ginger’s efficacy for the prevention of PONV have been equivocal. Some randomized studies and one small meta-analysis reported that ginger was not effective. However, a more recent and larger meta-analysis found that preoperative administration of ginger was more effective than placebo in 90 preventing PONV. Its effect on coagulation is ginger’s only known potential adverse effect in surgical patients. REFERENCES 1. Kaye AD, Kucera I, Sabar R. Perioperative anesthesia clinical considerations of alternative medicines. Anesthesiol Clin North America. 2004; 22:125-139. 2. Chaiyakunapruk N, Kitikannakorn N, Nathisuwan S, et al. The efficacy of ginger for the prevention of postoperative nausea and vomiting: A meta-analysis. Am J Obstet Gynecol. 2006; 194:95-99. 3. Eberhart LH, Mayer R, Betz O, et al. Ginger does not prevent postoperative nausea and vomiting after laparoscopic surgery. Anesth Analg. 2003; 96:995-998. 4. Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br J Anaesth. 2000; 84:367-371. 5. Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: Defining the risks. The Second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation. Reg Anesth Pain Med. 2003; 28:172-197. 6. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:605611. ITEM 89 Clinical scenario: As opposed to smaller prophylaxis dose, a patient is receiving a dose of 1 mg/kg enoxaparin every 12 hours for treatment of a deep venous thrombosis. The patient is scheduled for surgery under neuraxial anesthesia. In 1993 enoxaparin was the first low molecular weight heparin (LMH) approved by the Food and Drug Administration (FDA). Historically in Europe this drug has been administered in a once daily dose of 40 mg or less with no reported increase in the incidence of spinal hematoma associated with neuraxial anesthesia. However, in the United States—where enoxaparin is administered in a 30 mg dose every 12 hours, with the first dose administered as soon as possible after surgery—enoxaparin was rapidly reported to be associated with the development of spinal hematoma following neuraxial techniques. This eventually led to an FDA health advisory and “black-box” warning being issued in 1997. In April 1998 the American Society of Regional Anesthesia and Pain Medicine issued a consensus statement on neuraxial anesthesia in the anticoagulated patient. This statement was updated in 2003. The following are the society’s main conclusions regarding the timing of neuraxial anesthesia in patients receiving LMWH during the perioperative period. Preoperative LMWH Altered coagulation should be assumed in patients receiving LMWH An interval of at least 10-12 hours should occur between the last dose and performance of a neuraxial block A delay of at least 24 hours between the last dose and needle placement should occur in patients receiving doses used for treatment as opposed to doses used for prophylaxis If regional anesthesia is to be utilized, it would be particularly prudent to avoid needle placement during the peak of anticoagulant activity (ie, two hours after is administration) 91 Postoperative LMWH Single-injection and continuous catheter techniques may be safely used in patients receiving postoperative initiation of LMWH thromboprophylaxis. Management should be based on total daily dose, timing of the first postoperative dose and dosing schedule. Single daily dose o First postoperative LMWH dose should be administered six to eight hours postoperatively. o Second postoperative dose should occur no sooner than 24 hours after the first dose. o Indwelling neuraxial catheters may be maintained safely. However, catheters should be removed a minimum of 10-12 hours after the last dose of LMWH. Subsequent dosing should occur a minimum of two hours after catheter removal. Twice daily dosing o This dosing regimen may be associated with an increased risk of spinal hematoma. o Postoperatively, the first dose of LMWH should be administered no sooner than 24 hours, regardless of anesthetic technique, and only in the presence of adequate (surgical) hemostasis. o Indwelling neuraxial catheters should be removed prior to initiation of LMWH thromboprophylaxis. o If a continuous technique is selected, the epidural catheter may be left indwelling overnight and removed the following day, with the first dose of LMWH administered at least two hours after catheter removal. In accordance with these guidelines that patients receiving a treatment dose of 1 mg/kg enoxaparin every 12 hours should have neuraxial anesthesia and surgery delayed for at least 24 hours from the last dose. Waiting 12 hours from the last dose would be inadequate. The properties of LMWH differ from those of unfractionated heparin. LMWH, like unfractionated heparin, binds to antithrombin III and acts to inhibit activation of the coagulation factors Xa and IIa. However, LMWH has proportionally more anti-Xa activity than anti-IIa activity compared to unfractionated heparin. Monitoring of unfractionated heparin is achieved by measurement of an activated partial thromboplastin time (aPTT), which is prolonged during anticoagulation. LMWH does not cause a predictable increase in the aPTT, and this test is not a reliable measure of the degree of anticoagulation present during treatment. A prothrombin time is prolonged by deficiencies and abnormalities in, or inhibition of, factors I, II, V, VII, or X. It is typically used to monitor coagulation during warfarin use or hepatic dysfunction. However, it is not a reliable measure of anticoagulation in patients taking LMWH. REFERENCES 1. Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: Defining the risks (the Second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003; 28:172-197. Available online at: http://www.asra.com/consensus-statements/2.html. Accessed February 2007. 2. Fleisher LA. Evidence-Based Practice of Anesthesiology. Philadelphia: Elsevier Saunders; 2004:298-302. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2329, 2742-2743. 92 ITEM 90 Clinical scenario: A patient taking enoxaparin every 12 hours for treatment of a deep venous thrombosis is scheduled for surgery under neuraxial anesthesia. During placement of the epidural in this patient some blood is noted in the epidural catheter. The blood is cleared and does not reappear. The American Society of Regional Anesthesia and Pain Medicine (ASRA) addresses these issues in its consensus statement on regional anesthesia in the anticoagulated patient. The guidelines for the management of patients undergoing spinal and epidural blocks while receiving perioperative LMWH include the following: Monitoring of an anti-factor Xa level is not recommended because it is not predictive of the risk of bleeding and therefore not helpful in the management of patients undergoing neuraxial blocks. Antiplatelet or oral medications administered in combination with anticoagulant medications represent an additional risk of bleeding complications. Therefore, education and communication among the entire patient care team is necessary to avoid potentiation of the anticoagulant effects. Presence of blood during needle and/or catheter placement does not necessitate postponement of surgery. However, in this situation postoperative initiation of LMWH therapy should be delayed for 24 hours. Traumatic needle or catheter placement may signify an increased risk of spinal hematoma, and it is recommended that this issue be discussed with the surgeon. In the situation described in the scenario, the most appropriate management would be to proceed with the scheduled procedure but acknowledge that there may be an increased risk of spinal hematoma and postpone the administration of postoperative LMWH for 24 hours. The situation should also be discussed with surgical team members, confirming their awareness of the proposed management. REFERENCES 1. Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: Defining the risks (the Second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003; 28:172-197. Available online at: http://www.asra.com/consensus-statements/2.html. Accessed February 2007. 2. Fleisher LA. Evidence-Based Practice of Anesthesiology. Philadelphia: Elsevier Saunders; 2004:298-302. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2329, 2742-2743. ITEM 91 Clinical scenario: A 75-year-old female with a cardiac rhythm management device (CRMD) with defibrillation capabilities presents for an elective abdominal hysterectomy. Implantable cardiac defibrillators (ICDs) and pacemakers are now referred to as cardiac rhythm management devices (CRMD). As the complexity of CRMDs has increased, many of the previous methods of perioperative management no longer apply. This has led to an American Society of Anesthesiologists practice advisory in 2005 as well as other published guidelines for the appropriate perioperative management of patients with CRMDs. Some of the perioperative considerations are listed below (adapted from reference 1). 93 Preoperative Management Determine dependency on the pacing function of the device and establish the need for backup pacing support Ensure pacemaker/defibrillator interrogation by a competent individual shortly prior to the procedure and obtain a copy of the interrogation Consider replacement of any device or battery near its elective replacement period, particularly in patients scheduled to undergo major surgery Determine if a magnet mode is present and identify the magnet rate and rhythm For a defibrillator, disable antitachycardia therapy Program minute ventilation rate responsiveness off, if present Determine whether reprogramming the pacing function to an asynchronous mode or disabling rate-responsive functions would be advantageous Consider increasing the pacing rate to optimize oxygen delivery to tissues for major causes Intraoperative Management Closely monitor cardiac rhythm and peripheral pulse with pulse oximeter or arterial waveform Disable the “artifact filter” on the electrocardiography monitor Use bipolar electrosurgery; if possible, avoid monopolar The electrosurgery current return pad should be placed to prevent electricity from crossing the generator-heart area If monopolar must be used, pure cut is better than “blend” or “coag” Short bursts at irregular intervals using the lowest effective settings should be used Postoperative Management Equipment for emergency defibrillation must be immediately available The device should be interrogated postoperatively The device should be reprogrammed to optimum pacing rate as well as other parameters The patient with an ICD must be monitored until the antitachycardia therapy is restored The Food and Drug Administration issued an alert regarding those devices with minute ventilation rateadaptive capabilities because they can interact with monitoring and diagnostic equipment, resulting in the pacemaker pacing at its maximum programmed rate. When the CRMD features are disabled, rhythm monitoring needs to be maintained and external pacing pads should be in place. REFERENCES 1. Rozner MA. Controversies in perioperative pacemaker or defibrillator management. Fifty-seventh Annual Refresher Course Lectures and Basic Science Reviews: American Society of Anesthesiologists. 2006; No. 239. 2. Practice advisory for the perioperative management of patients with cardiac rhythm management devices: Pacemakers and implantable cardioverter-defibrillators: A report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Anesthesiology. 2005; 103:186-198. Available online at: http://www.asahq.org/publicationsAndServices/CRMDAdvisory.pdf. Accessed February 2007. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1546-1547. 94 4. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2329, 1421-1426. ITEM 92 Clinical scenario: A 75-year-old female with a cardiac rhythm management device (CRMD) with defibrillation capabilities presents for an elective abdominal hysterectomy. A magnet is placed over the generator of the CRMD. A cardiac rhythm management device (CRMD) with defibrillation capability (ICD) might be mistaken for a simple “pacemaker” by physical examination alone. The two different types of devices can generally be differentiated by chest radiograph based on its distinctive appearance (Figure 1 and Figure 2). Placement of a magnet over a CRMD will not always convert the device to a continuous asynchronous mode; responses can vary from no change to a loss of pacing altogether: In CRMDs with defibrillator capabilities, placement of a magnet may suspend or permanently disable tachydysrhythmia detection and therefore therapy. Some patients have had their antitachycardia therapy unintentionally disabled in this manner. CRMD brady-pacing functions also can respond to magnet placement differently; a few may change their mode or rate. Calling the manufacturer and/or interrogating the device remain the only ways to be confident as to what the magnet response will be. In addition, if a magnet is utilized, it is imperative the patient has his or her device reprogrammed, pacing parameters checked, and re-enabled postoperatively. Figure 1. Chest radiograph showing ICD. Note the presence of the two long defibrillating coils, sometimes described as “fuzzy leads.” 95 Figure 2. Chest radiograph showing simple pacemaker. REFERENCES 1. Rozner MA. Controversies in perioperative pacemaker or defibrillator management. Fifty-seventh Annual Refresher Course Lectures and Basic Science Reviews: American Society of Anesthesiologists. 2006; No. 239. 2. Practice advisory for the perioperative management of patients with cardiac rhythm management devices: Pacemakers and implantable cardioverter-defibrillators: A report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Anesthesiology. 2005; 103:186-198. Available online at: http://www.asahq.org/publicationsAndServices/CRMDAdvisory.pdf. Accessed February 2007. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:1546-1547. 4. McPherson CA, Manthous C. Permanent pacemakers and implantable defibrillators: Considerations for intensivists. Am J Respir Crit Care Med. 2004; 170:933-940. Epub 2004 Aug 5. Available online at: http://ajrccm.atsjournals.org/cgi/content/full/170/9/933. Accessed February 2007. 96 5. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2329, 1421-1426. ITEM 93 Clinical scenario: A 73-year-old male is undergoing general anesthesia for coronary artery revascularization. Shortly after induction, the monitor reveals a change in his electrocardiogram from normal sinus rhythm to the rhythm shown in Figure 1. Third-degree heart block (Figure 1) is a form of atrioventricular (AV) block in which the atrial impulses are not conducted to the ventricle, thus the atria and ventricles contract regularly but independently from each other. This high-grade conduction defect may also be called complete heart block, as there is complete AV dissociation. The QRS complex may be normal in appearance if the pacemaker site is in the AV node but will be widened if the pacemaker site is in the ventricle. Myocardial ischemia is usually the cause; and the hemodynamic manifestations result from inadequate perfusion from a slow ventricular rate. If the pacemaker site is in the ventricle, the rate will usually be slow, at or below 40 beats/min. Figure 1. Third-degree AV block. Second-degree AV block (Figure 2) occurs when some but not all of the atrial impulses are conducted to the AV node. It is further classified as Mobitz type I and Mobitz type II, depending on PR interval length. In second-degree Mobitz type I AV block (Figure 2), also called Wenckebach block, there is progressive lengthening of the PR interval until a beat is not conducted and no QRS is seen (a dropped beat). Figure 2. Second-degree AV block, Mobitz type I. In second-degree Mobitz type II AV block (Figure 3), the PR intervals remain the same length and a dropped beat occurs after a beat with a normal PR interval. Mobitz type II has a more serious prognosis as it may progress to complete heart block. 97 Figure 3. Second-degree AV block, Mobitz type II. First-degree heart block (Figure 4) occurs when all atrial impulses conduct to the AV node and into the Purkinje system but the PR interval is longer than 0.21 second. Figure 4. First-degree AV block. REFERENCES 1. Haro LH, Hess EP, Decker WW. Arrhythmias in the office. Med Clin North Am. 2006; 90:417438. 2. Perron AD, Sweeney T. Arrhythmic complications of acute coronary syndromes. Emerg Med Clin North Am. 2005; 23:1065-1082. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:14061409. 98 ITEM 94 Clinical scenario: A 73-year-old male is undergoing general anesthesia for coronary artery revascularization. Shortly after induction, the monitor reveals a change in his electrocardiogram for normal sinus rhythm to the following: The rhythm persists and after sternotomy, the patient’s blood pressure decreases to 70/40 mm Hg. Third-degree heart block is characterized by no atrial impulses being conducted through the atrioventricular (AV) node to the ventricles. Epicardial pacing wires would be indicated to increase the patient’s ventricular rate and cardiac output. Alternate choices of therapy would be the insertion of transvenous pacing wires or the administration of a direct beta1-agonist such as epinephrine, isoproterenol, or dobutamine for temporary support. If the patient’s sternum is already exposed, the placement of epicardial wires would be the most appropriate maneuver. Atropine is an anticholinergic agent that blocks parasympathetic (vagal) impulses to the heart. It may or may not be effective in patients with third-degree heart block. It may increase the atrial rate but will not affect the ventricular rate unless the atrial impulses are not conducted. Amiodarone, an antidysrhythmic drug with anti-adrenergic properties, will tend to suppress the ventricular rate, not increase it. Cardioversion is not indicated for third-degree heart block. REFERENCES 1. Gregoratos G. Indications and recommendations for pacemaker therapy. Am Fam Physician. 2005; 71:1563-1570. 2. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:11241125. ITEM 95 Clinical scenario: A 67-year-old, 81 kg woman with a history of asthma and hypertension has undergone a vaginal hysterectomy under general anesthesia. Induction was performed with fentanyl, propofol, and vecuronium (single 6 mg dose); sevoflurane was used for maintenance of anesthesia. Antibiotic prophylaxis was provided with clindamycin. No other drugs were administered. At the end of the 90minute procedure train-of-four (TOF) ulnar nerve stimulation produces four observed twitches without fade. Most clinicians employ several techniques to assure that their patients have adequate muscle strength to breathe, cough, and protect their airway. After the administration of nondepolarizing muscle relaxants, clinicians assess the probability of adequate muscle strength using dosing information (interval since last dose, total dose administered), clinical signs, and the response to a nerve stimulator. Commonly used nerve stimulation techniques include train-of-four (TOF), double burst, and sustained tetanus. 99 The TOF ratio (height of twitch 4:height of twitch 1) is considered the gold standard for accurate assessment of neuromuscular function. Unfortunately, true assessment of the TOF ratio requires a more complex technique than is commonly employed in the United States. Accurate measurement of the TOF ratio requires an accelerometer, usually placed over the thumbnail, to measure the excursion and speed of thumb movement in response to supramaximal stimulation of the ulnar nerve. Accelerometers have been shown to correlate with percent blockade of the neuromuscular junction or muscle contraction strength. The TOF Watch is such a device and is available in the United States. The vast majority of US anesthesiologists depend on a visual assessment of the TOF—with the presence of four twitches without fade being equated with adequate strength. When the observed TOF (oTOF) ratio is assessed to be 1 (twitch 1 = twitch 4) by the caregiver, the TOF ratio measured with an accelerometer (mTOF) is actually less than 0.6 in 48% of patients. Other studies have found the measured TOF ratio to be as low as 35% when four twitches are observed. An mTOF ratio of 0.7 has long been the lowest TOF accepted as safe for extubation and spontaneous ventilation. About half of all patients are at risk if the only technique to assess adequacy reversal is an oTOF ratio. Consequently, most clinicians also use clinical signs to confirm adequacy of reversal. Clinical signs commonly used to evaluate muscle strength are listed in Table 1. Either the five-second head or leg lift can be performed by more than half the patients when the mTOF ratio is less than 0.7. Only the ability to hold a tongue depressor steady in the mouth or demonstrate normal visual acuity are reliable indictors of an mTOF ratio greater than 0.7. Both measures are difficult to obtain in patients who are sedated by residual anesthetic agents. Likewise a handgrip is a poor measure of muscle strength since effort and motivation are major components of the response. The assessment of a “strong grip” is a subjective observation made by the caregiver. Table 1. Measured train-of-four ratio associated with common clinical assessment of muscle strength. Used with permission, from Murphy GS. Residual neuromuscular blockade: Incidence, assessment, and relevance in the postoperative period. Minerva Anestesiol. 2006; 72:97-109. Measured TOF Ratio Mean (range) Clinical Sign Head lift for 5 sec 0.6 (0.45-0.75) Leg lift for 5 sec 0.59 (0.5-0.65) Retained tongue depressor 0.86 (0.68-0.95) Visual disturbance absent 0.9-1.0 Grip strength Variable Volatile anesthetics have long been recognized to increase muscle relaxation from nondepolarizing neuromuscular blocking drugs. The effect can be demonstrated by comparing the time required to attain a designated mTOF ratio after neuromuscular reversal with a standard drug dosage. Comparisons are usually made between techniques using a volatile anesthetic such as sevoflurane and an intravenous agent such as propofol. At similar levels of muscle relaxation (eg, an oTOF count of 2), it will take two to three 100 times longer to achieve and mTOF ratio of 0.9 when the patient has received sevoflurane as compared to propofol. The more profound the muscle relaxation the greater the time required for adequate reversal. The decision to reverse a muscle relaxant is often made on the basis of the TOF count, oTOF ratio (presence or absence of fade), and clinical signs. Since these are unreliable indicators, reversal drugs should be administered unless mTOF ratio or clinical examination clearly demonstrates normal neuromuscular function. In patients who received a volatile anesthetic, the time to return of normal neuromuscular function is substantially prolonged, thus reversal is even more important. REFERENCES 1. Murphy GS. Residual neuromuscular blockade: Incidence, assessment, and relevance in the postoperative period. Minerva Anestesiol. 2006; 72:97-109. 2. Murphy GS, Szokol JW. Monitoring neuromuscular blockade. Int Anesthesiol Clin. 2004; 42(2):25-40. 3. Kim KS, Cheong MA, Lee HJ, et al. Tactile assessment for the reversibility of rocuroniuminduced neuromuscular blockade during propofol or sevoflurane anesthesia. Anesth Analg. 2004; 99:1080-1085. 4. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:421-452. ITEM 96 Clinical scenario: Reversal drugs are administered and the patient extubated. Immediately prior to extubation a small amount of fade was evident on train-of-four (TOF) stimulation. In the postanesthesia care unit (PACU), she is tachypneic and complains of difficulty breathing and swallowing. New information strongly suggests that what was once considered an adequate measured train-of-four (mTOF) ratio puts patients at significant risk of postoperative pulmonary complications. An mTOF ratio of 0.7 means the average patient is able to: maintain a head lift for five seconds or more maintain a leg lift for five seconds or more have a normal resting tidal volume have a vital capacity of 15-20 mL/kg be able to generate a negative inspiratory force of -20 cm H2O These clinical signs do not measure the ability of patients to adequately protect their airway, avert aspiration, and prevent pulmonary atelectasis. Current information suggests that patients with an mTOF ratio of 0.9 have a significantly reduced risk of complications compared to patients who have an mTOF ratio in the PACU of 0.7 (Table 1). 101 Table 1. Physiologic activity and measured train-of-four (mTOF) ratio where the activity returns to normal function. Returns at mTOF ratio of Activity Swallowing 0.8-0.9 Protect against aspiration 0.9 Speak clearly 0.9 Reliably maintain SPO2 0.9 The inability to prevent aspiration, cough, and perform a Valsalva maneuver leads to a significant increase in clinically important hypoxic episodes. Approximately 60% of patients with an mTOF of 0.7 on admission to the PACU have one or more episodes of hypoxia compared to 10% of patients who enter the PACU with an mTOF of 0.9. Patients with an mTOF less than 0.9 have an increased risk of requiring intensive care unit admission or developing pulmonary complications such as pneumonia. REFERENCES 1. Murphy GS. Residual neuromuscular blockade: Incidence, assessment, and relevance in the postoperative period. Minerva Anestesiol. 2006; 72:97-109. 2. Murphy GS, Szokol JW. Monitoring neuromuscular blockade. Int Anesthesiol Clin. 2004; 42(2):25-40. 3. Barash PG, Cullen BF, Stoelting RK. Clinical Anesthesia. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:421-452. ITEM 97 Clinical scenario: Over the next 90 minutes, the patient experiences several desaturation episodes. On a chest radiograph she has bilateral atelectasis. Her arterial blood gas analysis is PaO2 85 mm Hg, PaCO2 29 mm Hg, pHa 7.51, with an FIO2 of 0.4. You arrange for an intensive care unit bed. She complains of feeling weak and having difficulty swallowing. In patients with normal liver and renal function, it is very unusual for vecuronium to continue to cause muscle weakness more than three hours after a single 0.07 mg/kg dose. Other unknown medical conditions can cause a prolonged effect or increased sensitivity to nondepolarizing neuromuscular blocking drugs. Diseases that cause impaired neuromuscular function such as myasthenia gravis or Lambert-Eaton myasthenic syndrome may be asymptomatic until provoked by exposure to muscle relaxants. All acquired forms of neuromuscular disorders are very rare. Some, like familial hypokalemic periodic paralysis, would usually be recognized in childhood or young adulthood. The incidence of 102 myasthenia syndromes is about two per million population, occurring most frequently in women in their 20s and 30s and in men over 50. Impaired neuromuscular function with symptoms similar to myasthenia gravis may also occur in patients affected by other autoimmune disorders, hyperthyroidism, and lung cancer. Many patients with myasthenia gravis syndromes will present with symptoms similar to those seen in patients with inadequate reversal of neuromuscular blocking drugs: difficulty in visual accommodation (focus) difficulty with swallowing difficult with tongue coordination (speaking clearly) progressive difficulty with maintaining adequate respiratory effort The drugs most widely recognized to impair the function of the neuromuscular junction are antibiotics, aminoglycosides, clindamycin, and ciprofloxacin. Less frequently cited antibiotics that can cause impairment include tetracyclines and the topical antibiotics bacitracin, polymyxin B, and colistin. Other classes of drugs that can cause weakness include the antidysrhythmic drugs procainamide and quinidine as well as the beta-blockers propranolol and timolol. Disease states that reduce drug metabolism or renal clearance will prolong the duration of action. Significant liver disease and renal failure will increase the duration of action of steroidal muscle relaxants by approximately 30% (eg, an increase in elimination of half-life from 50 to 80 minutes). Significant liver disease is usually defined to occur when reductions in synthetic function cause prolonged prothrombin and partial prothrombin time. Genetic variations causing abnormal serum cholinesterase will reduce the metabolism of succinylcholine but a nondepolarizing muscle relaxant will not be affected. While it is possible the patient aspirated during the surgical procedure, the usual presentation of silent aspiration is a right lung infiltrate visible on chest radiograph and not bilateral atelectasis. Aspiration, if it occurs, is more likely in the PACU due to muscle weakness and the inability to protect her airway and cough. REFERENCES 1. O’Neill GN. Acquired disorders of the neuromuscular junction. Int Anesthesiol Clin. 2006; 44(2):107-121. 2. Atherton DP, Hunter JM. Clinical pharmacokinetics of the newer neuromuscular blocking drugs. Clin Pharmacokinet. 1999; 36:169-189. ITEM 98 Clinical scenario: A 70-year-old man with normal ventricular function undergoes uneventful coronary artery bypass surgery to the left anterior descending artery and the obtuse marginal artery. While still intubated in the intensive care unit (ICU), his central venous pressure increases from 7 mm Hg on arrival to 18 mm Hg over four hours. This is accompanied by decreases in his cardiac index and systemic blood pressure. There has been no significant chest tube output since the patient arrived in the ICU. Any patient undergoing heart surgery will be at risk for bleeding into the mediastinum during the immediate postoperative period. Unlike conditions that can produce pericardial effusions surrounding the entire heart, mediastinal bleeding can be localized and patchy. With asymmetric distribution of clot, equalization of diastolic pressures that is typically present with pericardial tamponade may not occur. Wherever the accumulation of blood occurs, it can compress the adjacent cardiac chambers, increasing 103 filling pressures (eg, central venous pressure). The patient will present with decreases in cardiac index and systemic blood pressure. Right ventricular failure is accompanied by increases in central venous pressure and decreases in cardiac index, however it is not the likely cause in this case. In a patient with previously normal right ventricular function, either massive fluid overload or ischemia in the distribution of the right coronary artery could cause sudden right ventricular failure. Both tamponade and right-sided heart failure should always be suspected in patients with significantly increased central venous pressure and decreased cardiac index in the immediate postoperative period. Hypovolemia could cause decreases in cardiac index and systemic blood pressure. Occurring alone, it would not cause increased central venous pressure. Myocardial ischemia in the distribution of the right coronary artery could cause right ventricular dysfunction with increased central venous pressure, decreased cardiac index, and decreased systemic blood pressure. In a patient having undergone coronary artery bypass grafting, this could occur if the graft to the right coronary artery or its tributary, the posterior descending artery, became thrombosed. This patient did not undergo a right-sided bypass grafting. REFERENCES 1. Heidecker J, Sahn SA. The spectrum of pleural effusions after coronary artery bypass grafting surgery. Clin Chest Med. 2006; 27:267-283. 2. St Andre AC, Del Rossi A. Hemodynamic management of patients in the first twenty-four hours after cardiac surgery. Crit Care Med. 2005; 33:2082-2093. 3. Miller RD. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:19661967. ITEM 99 Clinical scenario: A 70-year-old man with normal ventricular function undergoes uneventful coronary artery bypass surgery to the left anterior descending artery and the obtuse marginal artery. While still intubated in the intensive care unit (ICU), his central venous pressure increases from 7 mm Hg on arrival to 18 mm Hg over four hours. This is accompanied by decreases in his cardiac index and systemic blood pressure. There has been no significant chest tube output since the patient arrived in the ICU. It can be difficult to differentiate right heart failure from pericardial tamponade in the immediate postoperative period in a patient who has undergone cardiac surgery. The diagnosis should be made as quickly as possible since patients with active mediastinal bleeding can deteriorate rapidly. Pericardial tamponade (Figure 1) is a pathophysiologic condition that occurs when a cardiac chamber is compressed. In an intubated patient on mechanical ventilation with surgical dressings in the chest area, the acoustic windows are limited, making transthoracic echocardiography technically challenging and potentially preventing a definitive diagnosis (chamber compression). Transesophageal echocardiography will provide information about the function of both ventricles, fluid surrounding the heart, chamber compression, and ventricular filling patterns; thus, it is the diagnostic method of choice in this case. A 12-lead echocardiogram (ECG) can be obtained, but it cannot be used to diagnose cardiac chamber compression causing tamponade. In this situation, the ECG may exhibit a low voltage, however it would not show the electrical alternans pattern seen with other causes of pericardial tamponade involving large low-viscosity pericardial effusions. 104 Although a widened mediastinum will be evident on a chest radiograph with most causes of pericardial tamponade, the potential for localized compression of the heart by blood clot results in the possibility of hemodynamic consequences in the absence of diagnostic radiographic changes. Serum troponin concentration is a sensitive marker for myocardial damage, which is not suspected in this case. Figure 1. Mid-esophageal four-chamber view demonstrating a pericardial effusion and right atrial compression (arrow). Echocardiographic features suggestive of tamponade include right atrial compression or inversion, right ventricular diastolic collapse, changes in right and left ventricular filling patterns with respiration, and inferior vena cava plethora. REFERENCES 1. Piazza G, Goldhaber SZ. The acutely decompensated right ventricle: Pathways for diagnosis and management. Chest. 2005; 128:1836-1852. 2. Chiles CD, Menon V. Echocardiographic tamponade in severe left ventricular dysfunction: The impact of small pericardial effusion and the absence of pulsus paradoxicus. J Am Soc Echocardiogr. 2004; 17:78-79. 3. Otto CM. Textbook of Clinical Echocardiography. 3rd ed. Philadelphia: WB Saunders; 2004; 261269. 105 ITEM 100 Clinical scenario: A 70-year-old man with normal ventricular function undergoes uneventful coronary artery bypass surgery to the left anterior descending artery and the obtuse marginal artery. While still intubated in the intensive care unit (ICU), his central venous pressure increases from 7 mm Hg on arrival to 18 mm Hg over four hours. This is accompanied by decreases in his cardiac index and systemic blood pressure. There has been no significant chest tube output since the patient arrived in the ICU. The patient has become increasingly tachycardic to 120 beats/min with further decline in cardiac index. In the setting of pericardial tamponade, tachycardia reflects activation of the patient’s sympathetic nervous system in response to decreased organ perfusion. With tamponade, systemic perfusion is optimized by maintaining preload, afterload, tachycardia, and contractility. The definitive management is to return to the operating room and perform a mediastinal exploration to evacuate the blood and clot in the patient’s chest. Temporizing measures prior to returning the patient to the operating room include intravenous fluid administration (including blood and blood products) and support of contractility, vasomotor tone, and heart rate. Although a beta-blocker such as esmolol would decrease the patient’s heart rate, it would also decrease the cardiac index. In this situation, there is a mechanical cause for decreased cardiac filling, and slowing the heart rate will not improve cardiac filling and in fact could be very detrimental. Administering diltiazem could cause further hemodynamic compromise by decreasing the heart rate and cardiac index. As the obstruction to cardiac filling is mechanical, slowing the heart rate will not improve cardiac filling. Diuresis would not be indicated, as the increased central venous pressure reflects venous congestion in the systemic veins and not the patient’s intracardiac volumes, which are low. REFERENCES 1. St Andre AC, Del Rossi A. Hemodynamic management of patients in the first twenty-four hours after cardiac surgery. Crit Care Med. 2005; 33:2082-2093. 2. Troianos CA. Anesthesia for the Cardiac Patient. St. Louis: Mosby; 2002: 317-338. 106 IN-TRAINING TAXONOMY The Joint Council on In-Training Examinations of the American Board of Anesthesiology and the American Society of Anesthesiologists have prepared a Content Outline. It is used by the Joint Council to code the questions of the In-Training Examination for subject area. Using this Content Outline, the Editorial Board of the Anesthesiology Continuing Education Program provides one or more codes relating to the theme(s) of each question of the ACE Program. These codes will be tallied and printed in each volume. This index of Content Outline Codes may be of some use in identifying specific content areas within the ACE Program. The Content Outline was last revised in 2006. Please note that the current issue (4A and 4B) of the ACE Program continues to use the 2003 version of the Content Outline available at http://www.asahq.org/publicationsAndservices/Content%20Outline%20REV%202003.pdf. Beginning with issue 5A, the 2006 revised Content Outline will be used. 107 108 ALPHANUMERICAL LIST OF CONTENT OUTLINE CODES IN-TRAINING TAXONOMY (item numbers in bold) I. BASIC SCIENCES A. Anatomy: B. Physics, Monitoring, and Anesthesia Delivery Devices: 1, 11, 28, 29, 38, 50, 51, 68, 69, 70, 93, 94, 95, 96, 97 C. Mathematics: 35, 74 D. Pharmacology: 2, 5 ,7, 9, 10, 14, 20, 23, 24, 26, 27, 39, 48, 56, 57, 59, 67, 87, 88, 96, 97 II. CLINICAL SCIENCES A. Anesthesia Procedures Methods, and Techniques: 3, 4, 9, 12, 36, 40, 44, 45, 65, 74, 75, 82, 83, 89, 90 III. ORGAN-BASED BASIC AND CLINICAL SCIENCES A. Respiratory System: 6, 31, 60, 64, 69 B. Cardiovascular System: 6, 18, 25, 32, 36, 37, 43, 51, 58, 61, 63, 66, 71, 73, 81, 82, 83, 91, 92, 93, 94, 98, 99, 100 C. Central and Peripheral Nervous Systems: 8, 23, 24, 49, 53, 54, 62, 76, 77, 87 D. Gastrointestinal/Hepatic: 16, 42 E. Renal Urinary: 52 F. Endocrine/Metabolic: 34 G. Hematology: 17, 72, 84, 85, 86 IV. CLINICAL SUBSPECIALTIES A. Painful Disease States: 4, 14, 15, 41, 46, 47, 53, 78, 79 B. Pediatric Anesthesia: 30, 55 C. Obstetrical Anesthesia: 8, 21, 33, 65, 80, 81 D. Otolaryngology (ENT) Anesthesia; Airway Endoscopy; Microlaryngeal Surgery; Laser Surgery; Hazards, Complications; Jet Ventilation: E. Anesthesia for Plastic Surgery, Liposuction: F. Anesthesia for Laparoscopic Surgery; Cholecystecomy; Gynecologic Surgery; Gastric Stapling; Hiatus Hernia Repair; Anesthetic Management; Complications: G. Ophthalmologic Anesthesia, Retrobulbar and Peribulbar Blocks; Open Eye Injuries: 76, 77 H. Orthopedic Anesthesia; Tourniquet Management, Complications, Regional vs General Anesthesia: I. Trauma, Burn Management, Mass Casualty, Biological/Chemical Warfare: J. Ambulatory Anesthesia: K. Geriatric Anesthesia/Aging: 22 V. SPECIAL PROBLEMS OR ISSUES A. Electroconvulsive Therapy: B. Organ Donors: C. Radiologic Procedures; CT Scan; MRI: D. Ethics, Practice Management, Medicolegal Issues: 13, 19 109