Lab 8 lab report
advertisement

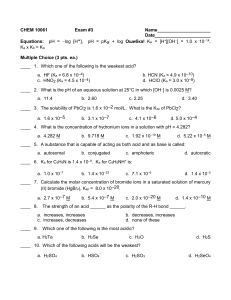
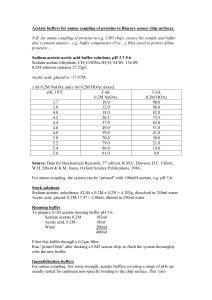
Experiment 8 pre-lab 1. Ken McFarland “Buffers”, CHEM 1130, TA - Nastaran Marzijarani, Section 108, Room 1871, Will Brubacher, Thursday 2:00 pm 2. Prelab Questions: Consider a buffer made by mixing equal amounts of acetic acid and sodium acetate. Refer to equation 8-1 and a. Describe the relative amounts of each of the three compounds present in the buffer solution: As the Ksp is much less than one, the acid does not dissociate much. Most of the acid is HOAc, with relatively little H+ or OAc-. b. Predict what will happen to the concentrations of each of the three components if a small amount of HCl is added: The [HOAc] will increase, and the [H+] and [OAc-] will decrease. c. Predict what will happen if a small amount of NaOH is added: the [HOAc] will decrease, and the [H+] and [OAc-] will increase. 2. Generate an outline of a procedure for preparing 100 mL of an acetic acid/acetate buffer. a. Add equal amounts of HOAc and NaOAc solutions, and DI water if necessary, to produce a 100 Ml solution. 3. If a buffer is prepared using equal amounts of HOAc and NaOAc predict what the pH of that solution will be. a. The pH of the buffer solution will be the pKa, or 4.7 4. If the buffer is made up to be 1.0 M in acetic acid and 1.0 M in sodium acetate, predict how much acid will be required to change the Ph of 100 mL of the buffer by 0.1 pH units a. 8.7 mL of 1 M acid 3. Data: See attached data sheets. 4. Discussion: Both of the solutions should have required 11.5 mL of titrant to change the pH by 0.1 (math shown on attached data sheet), however our results were off. It took only 5.5 mL of HCl to reduce the pH by 0.1, while it took 16 mL of NaOH to increase the pH by 0.1. This is a percent error of 52.2% for the acid, and 39.1% for the base. The lab also involved creating buffer solutions with pH’s of 4.50, and 5.00 using sodium acetate and acetic acid. (Work shown on attached data sheet.) We created solutions with a pH of 4.5 exactly and 5.18, a percent error of 0% and 3.6%.